can anyone explain to me about how to find serial dilution? thanks in advance. i'm still unsure about them please anyone please help me  it's very urgent! if anyone respond to this i'd be eternally grateful and be sure to remember you in my prayers, amin~
it's very urgent! if anyone respond to this i'd be eternally grateful and be sure to remember you in my prayers, amin~
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
explanation needed for biology practical
- Thread starter eyamwir
- Start date
- Messages
- 188
- Reaction score
- 9
- Points
- 28
The technique used to make a single dilution is repeated sequentially using more and more dilute solutions as the "stock" solution. At each step, 1ml of the previous dilution is added to 9ml of distilled water. Each step results in a further 10-fold change in the concentration from the previous concentration.
The values shown in the tubes are the amount (in ml) of the stock solution present in each ml of the dilute solution.
The dilution of the original stock solution is sho
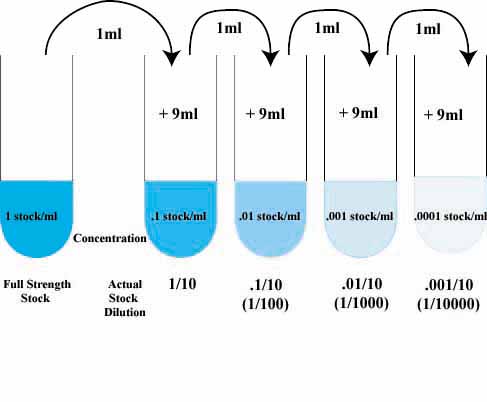 wn below the tubes.
wn below the tubes.
The values shown in the tubes are the amount (in ml) of the stock solution present in each ml of the dilute solution.
The dilution of the original stock solution is sho

- Messages
- 188
- Reaction score
- 9
- Points
- 28
Hope it helps! 
Hope it helps![/quote
so does that mean distilled water is added to the test tube for every new test tube? and i don't get it with the ml. :s sorry