- Messages
- 561
- Reaction score
- 733
- Points
- 103
thanksHeres why !
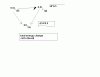
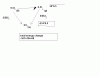
First the answer we are looking for !
PV=(2/2)RT
A- total moles = 2/4 =0.5
and so the overall equation is !
PV=0.5nRT which is impractical !
B- total moles = 4/4 =1
P(V/2)=nRT the volume if it was in A,then it would have been right but thats not the case !
C- remember moles for different compounds or elements cant be found by one equation u have to find them individually thats one basic mistake !
total moles = 1/2(H2) + 2/4 (D2) =1
PV=nRT (the correct answer )
D- total moles = 2/2 + 1/4 = 1.25
2PV=1.25nRT (not possible)