- Messages
- 243
- Reaction score
- 198
- Points
- 43
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
PLEASE HELP!!! DESPERATE HERE! MAY U B BLESSED $ HELPIN!!!
View attachment 25901
The idea of temperature is that thermal energy will be transferred from a body/object/system with a higher temperature to a body/object/system of a lower temperature, i.e. thermal energy flows from a body with high temperature to a body with a lower temperature.
If body A and body B were at the same temperature, there would be no energy flow between them. If body B and C were at the same temperature, no energy would flow between them (from one to the other).
However, if what the zeroth law says is false, then energy will flow between body A and body C, while there would be no energy flow between body B and body C, but since body B is at the same temperature as body A, you're transferring energy in one situation and not doing so in another, practically identical situation!
In a way, the concept of temperature and the zeroth law come together to ensure that conservation of energy is not defied - again, in one situation you'll be transferring energy and in the other, you'll not!
Hope this helped!
Good Luck for all your exams!
Stuck badly please help!! May u be blessed for helping! Dnt knw ans to it!
1. Describe the changes to the kinetic energy, the potential energy and the total internal energy of a block of ice as:
i. it melts at 0oC
ii. the temperature of water rises from 0oC to room temperature.
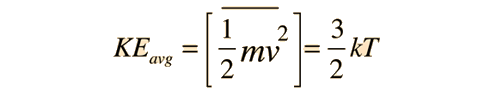
The basic idea of temperature is that it is a measure of the kinetic energy component of a substances internal energy, as this equation shows:
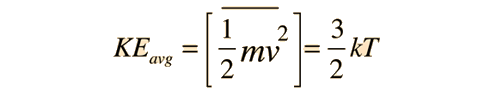
(Image taken from http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/kintem.html)
Going down that page, you can find a more visible form of this equation and some extra information about it.
So the greater the temperature, the greater the average kinetic energy of the particles in the substance.
When a block of ice melts, a change of state is occurring, so there is no change in temperature. If there is no change in temperature, there is no change in the average kinetic energy of the particles in the substance, so there is no change in the internal kinetic energy of the subtance.
However, when the process of melting occurs, the energy supplied/taken in by the ice is used to break bonds between the water molecules in the ice (mostly Hydrogen Bonds), so the molecules are more free to move apart. This can be seen since liquid water can flow, while solid ice cannot change its shape in this manner. When molecules in a substance move farther apart, their internal potential energy increases (just like lifting a mass above the surface of the Earth - the separation between the mass and the Earth increases, and thus so does its kinetic energy).
So, the bottom line for (i) is that the internal kinetic energy remains constant, internal potential energy increases and thus internal energy on a whole increases.
For (ii), the energy supplied to increase the temperature of the now liquid water from 0 degrees celsius to room temperature doesn't go some into breaking intermolecular bonds - the water does not flow more easily/become more compressible (or at least, if these effects are there, they are not so noticeable!), etc during this increase in temperature, so we can safely say that the change in internal potential energy is practically, for all intents and purposes, zero.
But since the temperature is increasing, and temperature is a measure of the average internal kinetic energy of the particles in the substance, the internal kinetic energy is increasing!
Therefore, for (ii), it can be said that the internal potential energy doesn't increase but the internal kinetic energy does increase. Due to this, the overall internal energy does increase for this process.
Sorry for making it so long, hope it's okay!
Hope this helped!
Good Luck for all you exams!
For almost 10 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now