- Messages
- 1,476
- Reaction score
- 1,893
- Points
- 173
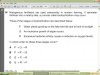
*Which one these always applies to a nucleophile?
A) It attacks a double bond.
B) It has a lone pair of electrons.
C) It has a single atom.
D) It is negatively charged.
Why its not D?
It is not D because there are some nucleophiles which are not negatively charged, for example:
NH3 (ammonia) is a nucleophile as it has lone pair of electrons but it is not negatively charged...