- Messages
- 5
- Reaction score
- 19
- Points
- 3
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_s05_qp_1.pdf
Q 24....anyone? Thank you
Q 24....anyone? Thank you
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s05_qp_1.pdf
can somebody plz explain some questions from m/j/5
Q1-C
Q2-B
Q13-B
Q14-C (but D is also correct the sum of first two ionization energies for ca is greater)
Q38-A
ANY KIND OF HELP WOULD BE APPRECIATED
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w10_qp_12.pdf Q10 ans B , Q18 ans b , Q20 ans C , Q25 ans D and Q38 ans A Please help if u can
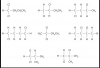
Sorry you are right the answer to 38 is C but in Q20 there are 3 carbon atoms not 4Q10. Graphite is made up of sheets of hexagonal rings, the angle among the carbon atoms are 120 degrees.
Q18.
A. (Wrong) Br2 is non-polar molecule (ID-ID intermolecular attractions), should be soluble in non-polar solvents.
B. (Correct) Br2 molecule mass is 80 x 2 = 160, which is heavier than air (Mr approximately 30).
C. (Wrong) Br2 vapourise easily (low boiling point) due to ID-ID intermolecular attraction.
D. (Wrong) Gaseous bromine is reddish brown.
Q20. Questions like these are quite time consuming if many atoms are involved. Might be more sensible to spend time on other questions first during the actual exam.
View attachment 44361
Q25. First 3 reactions require heating. The 4th one is reaction of 2,4-DNPH with carbonyl, which can be carried out at room temperature.
38.
I seemed to have 2 and 3 as the answer. The answer provided was A?
Is 39 C?http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w02_qp_1.pdf
Can anybody please explain questions 5,13,23,36 and 39?
The problems are as follows:
In 5 why can't A be correct as it is symmetrical.
In 13 why can't A(Mg) be the answer as when it dissolves the pH is around 6.5 which suggests hydrolysis.
In 23 why can't D(180) be the answer as the chain is like -C-C-C-C-C- which shows the C-C-C bond to be 180.
In 36 why is 2(NH3 behaves as a base) is correct.
In 39, actually I don't get a single point!
Thanks in advance and please try these questions out if possible as they are confusing and they might even help you out!
Is 39 C?
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w02_qp_1.pdf
Can anybody please explain questions 5,13,23,36 and 39?
The problems are as follows:
In 5 why can't A be correct as it is symmetrical.
In 13 why can't A(Mg) be the answer as when it dissolves the pH is around 6.5 which suggests hydrolysis.
In 23 why can't D(180) be the answer as the chain is like -C-C-C-C-C- which shows the C-C-C bond to be 180.
In 36 why is 2(NH3 behaves as a base) is correct.
In 39, actually I don't get a single point!
Thanks in advance and please try these questions out if possible as they are confusing and they might even help you out!
Thanks mate!To add Br2 to an alkene, the two Br atoms must end up on carbons next to each other.
It can be done by common sense tooCan anyone help me with this question?
11 0.144 g of an aluminium compound X react with an excess of water, to produce a gas. This gas
burns completely in O2 to form H2O and 72 cm3 of CO2 only. The volume of CO2 was measured at
room temperature and pressure.
What could be the formula of X?
[C = 12.0, Al = 27.0; 1 mole of any gas occupies 24 dm3 at room temperature and pressure]
A Al 2C3 B Al 3C4 C Al 4C3 D Al 5C3
Hi all,
in view of the lack of solutions for past year MCQ papers, I've attempted to create a youtube playlist to explain the answers.
It is a long and laborious process, it is not possible for me to cover a large number of practice papers before your exams on 10 June, I view it as more of a long term project for future batches.
I'm sure that there will be mistakes in my videos as they are done in 1 long sitting, so feel free to point out any mistakes or clarify concepts.
If the videos prove to be useful, I will slowly add on to them in the future.
Here is the link to the June 2013 P11.
http://www.youtube.com/playlist?list=PL4Jmce8VJnNXQDPOYEr7j99fO9nIy55eh
I'll create start a thread elsewhere so the video links can be neatly maintained over time.
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now