- Messages
- 164
- Reaction score
- 193
- Points
- 53
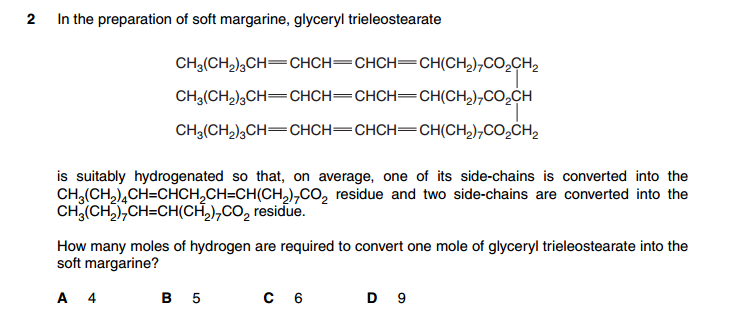
I solved this question by considering each option one at a time.http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s12_qp_11.pdf
Number 9?? Answer B
Anyone please??
Option B)
.......2P <=> 2Q + R
I.......2...........0.......0
C....-2x........+2x....+x
E....2-2x........2x......x
2-2x + 2x + x
= 2 + x