-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Help with this one plz, ans is B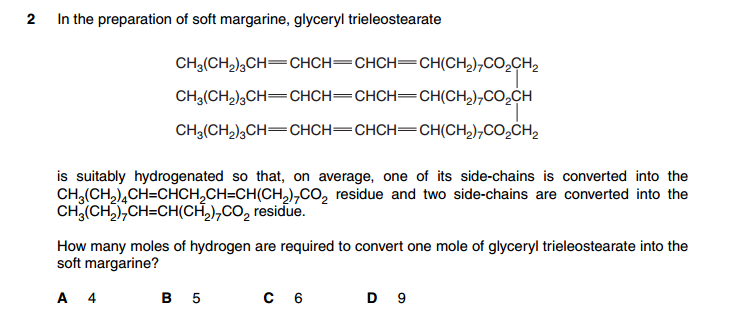
You can check out post #9267 on page 464
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w12_qp_34.pdf
Can someone please explain me Q1 part c i, how to calculate the initial concentration. Thanks.
Expt 1: moles of H2O2 = original conc x original vol = 0.23 x 0.05 =0.0115 mol
conc of H2O2 at start of experiment 1 = moles of H2O2/volume of solution = 0.0115/0.130 = 0.0885 mol dm-3
- Messages
- 49
- Reaction score
- 68
- Points
- 18
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w06_qp_1.pdf
Plz help me with 3 questions from o/n/6
Q9-C
Q11-B
Q25-B
Thanku
Plz help me with 3 questions from o/n/6
Q9-C
Q11-B
Q25-B
Thanku
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Video for June 2013 Paper1_2 is uploaded
http://www.youtube.com/playlist?list=PL4Jmce8VJnNWSchiyYdNNlLsHJlIvfZ35
http://www.youtube.com/playlist?list=PL4Jmce8VJnNWSchiyYdNNlLsHJlIvfZ35
- Messages
- 10
- Reaction score
- 25
- Points
- 23
AS SALAMO ALAIKUM
can some one plzz help me with q 28 , 37 & 39
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w04_qp_1.pdf
can some one plzz help me with q 28 , 37 & 39
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w04_qp_1.pdf
- Messages
- 8,521
- Reaction score
- 34,851
- Points
- 718
28)AS SALAMO ALAIKUM
can some one plzz help me with q 28 , 37 & 39
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w04_qp_1.pdf
1 u know is right
2) when two ethane radicals combine
3) when combination of ethane radical is chlorinated
37)
Remember the termination step. Two free radicals join to form a molecule. Break this molecule CH3CHCl-CHClCH3 into this CH3*CHCl and *CHClCH3. These are two free radicals formed in the propagation step. Whenever these questions come. Break the central bond and check if two free radicals are formed.
39)
♣♠ Magnanimous ♣♠
- Messages
- 70
- Reaction score
- 171
- Points
- 43
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s12_qp_11.pdf
Can someone explain Q 2, 9, 13 ,14, 22 and 36
Thanks!
Can someone explain Q 2, 9, 13 ,14, 22 and 36
Thanks!
- Messages
- 528
- Reaction score
- 1,241
- Points
- 153
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s11_qp_32.pdf
HELP!!! how do we calculate the enthalpy change??!! :$
Question 2 b (iii)
HELP!!! how do we calculate the enthalpy change??!! :$
Question 2 b (iii)
- Messages
- 6,392
- Reaction score
- 27,001
- Points
- 698
it's simple!AS SALAMO ALAIKUM
can some one plzz help me with q 28 , 37 & 39
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w04_qp_1.pdf
see 39) when there is an OH the reaction can occur with sodium metal and that is given in the book which means tatement 1 is CORRECT!
statement 3) overall molecular formula is C5H12O4 so it cannot be CH3O which means this is wrong and due to this statement 2 is also eliminated so answer is D
- Messages
- 528
- Reaction score
- 1,241
- Points
- 153
Guys.. Any help???http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s11_qp_32.pdf
HELP!!! how do we calculate the enthalpy change??!! :$
Question 2 b (iii)
Plus question 3 (b) as well!! The ion part! Pleaaasseeeee!!!
- Messages
- 6,392
- Reaction score
- 27,001
- Points
- 698
Thought blocker dude! I need your help in one thing of enthalpy calculation if online do reply?
OR ANY ONE CAN REPLY IF AVALIABLE!
OR ANY ONE CAN REPLY IF AVALIABLE!
- Messages
- 8,521
- Reaction score
- 34,851
- Points
- 718
Wait, I have loads of work... Will come to your house, or will do it tomorrowThought blocker dude! I need your help in one thing of enthalpy calculation if online do reply?
OR ANY ONE CAN REPLY IF AVALIABLE!
- Messages
- 6,392
- Reaction score
- 27,001
- Points
- 698
well i am not sure about 9 amd 11http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w06_qp_1.pdf
Plz help me with 3 questions from o/n/6
Q9-C
Q11-B
Q25-B
Thanku
25) it should be B na bcoz there are 3n carbon and 6n Hydrogen so if we balance the equation we get 9 moles of oxygen on the product side so for balancing the moles of oxygen in reactact obviously it should 4.5 because it already O2 so simple calculation for verfication --> 4.5 *2 ==> 9 so the overall equation is balanced
I hope you got it!
- Messages
- 6,392
- Reaction score
- 27,001
- Points
- 698
bro it is simple calculation needed! you know na my maths probs so. and this is enthalpy calculations! it will hardly take 2 min for youWait, I have loads of work... Will come to your house, or will do it tomorrow
- Messages
- 8,521
- Reaction score
- 34,851
- Points
- 718
Post it, if I would be free, I'd solve it. Go on!bro it is simple calculation needed! you know na my maths probs so. and this is enthalpy calculations! it will hardly take 2 min for you
- Messages
- 6,392
- Reaction score
- 27,001
- Points
- 698
As you know that for finding entalpy we substract ==> product - reactantPost it, if I would be free, I'd solve it. Go on!
than solve this for me .. hey me bhu gaya kiska doubt tha
i got sweet sugar post ques 9 cmon bro do it!
- Messages
- 8,521
- Reaction score
- 34,851
- Points
- 718
Link ?As you know that for finding entalpy we substract ==> product - reactant
than solve this for me .. hey me bhu gaya kiska doubt tharuk do min ab
i got sweet sugar post ques 9 cmon bro do it!