-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Bio URGENT help (dilution)
- Thread starter fatema
- Start date
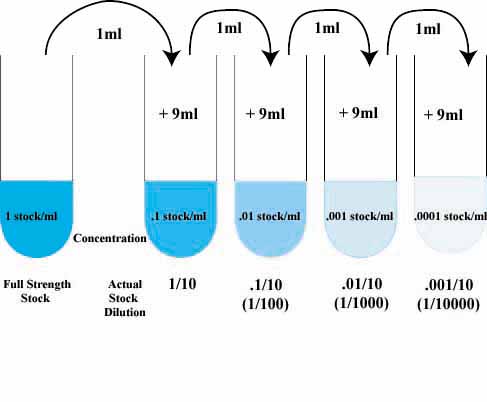
For serial dilution the common formula used is C1V1=C2V2;
where C1 is the concentration of solution provided and V1 volume of solution to be used
while C2 is the conc of solution to be made and V2 is the total volume of new solution(i.e. volume of V1 + Distilled water-DW- added)
Consider you are provided with 5% sugar. In order to carryout serial dilution and make a resulting solution of 2.5%, take 5ml of 5% solution add 5ml of DW. This makes a 10ml of 2.5% sugar solution
According to equation;
C1V1=C2V2
5x5=2.5x10
where C1 is the concentration of solution provided and V1 volume of solution to be used
while C2 is the conc of solution to be made and V2 is the total volume of new solution(i.e. volume of V1 + Distilled water-DW- added)
Consider you are provided with 5% sugar. In order to carryout serial dilution and make a resulting solution of 2.5%, take 5ml of 5% solution add 5ml of DW. This makes a 10ml of 2.5% sugar solution
According to equation;
C1V1=C2V2
5x5=2.5x10
