- Messages
- 872
- Reaction score
- 894
- Points
- 103
Isn't 37 c?? What is it?http://papers.xtremepapers.com/CIE/Cambridge International O Level/Chemistry (5070)/5070_s12_qp_11.pdf
Q.34. How is it 3? :/
And 37 also
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Isn't 37 c?? What is it?http://papers.xtremepapers.com/CIE/Cambridge International O Level/Chemistry (5070)/5070_s12_qp_11.pdf
Q.34. How is it 3? :/
And 37 also
I know it is C, but how?Isn't 37 c?? What is it?
The hydrocarbon and the total golyme of products are in a ratio 1:7. for 1 mole of the hydrocarbon, the total vilume of the products (both water and co2) is 7 moles, so the answers c. Is 40 A?I know it is C, but how?
And could you do no. 40?
The hydrocarbon and the total golyme of products are in a ratio 1:7. for 1 mole of the hydrocarbon, the total vilume of the products (both water and co2) is 7 moles, so the answers c. Is 40 A?
The CH3 or Methyl group is attached alternatively in many ways in the polymer, it affects the melting point and crystalline structure of the polymer.Could someone please answer this. Why are alternate carbons bonded to ch3 groups in the structure of polypropene?
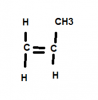
What if there's no alkyl group attached to the monomer?The CH3 or Methyl group is attached alternatively in many ways in the polymer, it affects the melting point and crystalline structure of the polymer.
If you are asking why, then consider the monomer, i.e. propene, it has a carbon-carbon double bond with 3 hydrogen atoms and a methyl group attached, when the polymer forms (by addition), there is a series of propene molecules that add up to each other, this forms a single horizontal carbon chain connected to hydrogen and methyl group alternatively. Here's a picture of how propene is drawn to form a polymer.
View attachment 26150
There are quite a few, which book do you have?Also, could someone please post all the procedures of the industrial processes
That's not possible for propene, the reason is that unless the monomer structure is like the picture I posted above, the polypropene CANNOT form since a repeat structure would not be formed.What if there's no alkyl group attached to the monomer?
Chem insight and this other book but the second one isn't useful at allThere are quite a few, which book do you have?
Oh, I don't have insights; I'll post the procedures after tomorrow's paper.Chem insight and this other book but the second one isn't useful at all
Does polymethene exist? So if the unsaturated hydrocarbon doesn't have an alkyl group, it can't have a repeat unit? What if the repeat unit is h-c-h-h-?That's not possible for propene, the reason is that unless the monomer structure is like the picture I posted above, the polypropene CANNOT form since a repeat structure would not be formed.
Okay thanksOh, I don't have insights; I'll post the procedures after tomorrow's paper.
Methene doesn't exist so how can Polymethene exist?Does polymethene exist? So if the unsaturated hydrocarbon doesn't have an alkyl group, it can't have a repeat unit? What if the repeat unit is h-c-h-h-?
Ohm's law states that at constant temperature and pressure, all ohmic conductors' voltage is directly propotional to the current (i.e. R=V/I), where R is the constant/resistance.hello ppl! if the questions asks us to state ohm's law, do we just right R=V/I? or r we supposed to explain something? (the question was for 2 marks for some reason) :s (IGCSE physics btw)
ook! thnxx alot bro...wow...amazing definition...Ohm's law states that at constant temperature and pressure, all ohmic conductors' voltage is directly propotional to the current (i.e. R=V/I), where R is the constant/resistance.
MashaAllah, I did it:http://papers.xtremepapers.com/CIE/Cambridge IGCSE/Physics (0625)/0625_w10_qp_33.pdf
u guys...Q.10 part (a) please...ts really difficult....nd the amazing examiners didnt know how to phrase it in the mark scheme...:|
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now