-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
bond angle
- Thread starter hassam
- Start date
Bond pair / lone pair / bond angle / shape
2 0 180 linear
2 1 105 non linear
2 2 105 non linear
3 0 120 trigonal planar
3 1 107 trigonal pyramidal
3 2 90 T shaped
4 0 109.5 tetrahedral
4 1 - distorted tetrahedral
4 2 90 Square planer
5 0 90/120 Square pyramidal
5 1 90 trigonal bipyramidal
6 0 90 Octahedral
You can figer out shape and bond angle from the table
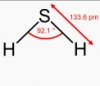
eg. H2S ....bond pair 2 and lone pair 2...thus bond angle is 105(104.5) nd the shape is nonlinear (bent or V shaped)
2 0 180 linear
2 1 105 non linear
2 2 105 non linear
3 0 120 trigonal planar
3 1 107 trigonal pyramidal
3 2 90 T shaped
4 0 109.5 tetrahedral
4 1 - distorted tetrahedral
4 2 90 Square planer
5 0 90/120 Square pyramidal
5 1 90 trigonal bipyramidal
6 0 90 Octahedral
You can figer out shape and bond angle from the table
eg. H2S ....bond pair 2 and lone pair 2...thus bond angle is 105(104.5) nd the shape is nonlinear (bent or V shaped)
- Messages
- 133
- Reaction score
- 47
- Points
- 38
The larger the central atom is the less the hydrogens have to spread out (because the electron repulsions are smaller) and the smaller the resulting angle.
This explaination seems quite controversial to the A level Chem content, but u all know there are AlwAys some exceptions in all sciences....;p
This is an extract from another forum:
"You cannot extrapolate the VSEPRT to other molecules such as PH3 simply because the greater size of the phosphorus atom basically affects the inter electron pair repulsion.
It's all very well to say that NH3 is 107º therefore PH3 will be as also - it just isn't.. there are other factors to consider such as the polarised nature of the N-H bond when compared to the P-H bond. The H-P-H bond angle is 93.7º even though it is pyramidal
This also applies to H2S and H2O - they do not have the same bond angle.
The VSEPRT does not predict bond angles , rather it explains them based on some rather simplistic and easily understood "rules"
Just learn the simple molecules H2O, NH3, CH4, XeF4, etc but do not be at all surprised by seemingly similar molecules with very different bond angles."
This explaination seems quite controversial to the A level Chem content, but u all know there are AlwAys some exceptions in all sciences....;p
This is an extract from another forum:
"You cannot extrapolate the VSEPRT to other molecules such as PH3 simply because the greater size of the phosphorus atom basically affects the inter electron pair repulsion.
It's all very well to say that NH3 is 107º therefore PH3 will be as also - it just isn't.. there are other factors to consider such as the polarised nature of the N-H bond when compared to the P-H bond. The H-P-H bond angle is 93.7º even though it is pyramidal
This also applies to H2S and H2O - they do not have the same bond angle.
The VSEPRT does not predict bond angles , rather it explains them based on some rather simplistic and easily understood "rules"
Just learn the simple molecules H2O, NH3, CH4, XeF4, etc but do not be at all surprised by seemingly similar molecules with very different bond angles."
- Messages
- 133
- Reaction score
- 47
- Points
- 38
Check this out @ http://en.wikipedia.org/wiki/Phosphine
See the H-P-H bond angle given there as 93.5 degrees
This is anoth3r contradiction jux like H2S. The truth is that the cambrigde is afraid of facing the reality and tends to teach its students what is easy to understand, even if it's against the reality. Lemme elaborate wid a good example:- When we are young, we are told by our elders that the Earth is ROUND but when we grow older we find that actually it ain't ROUND, rather it's SPHERICAL.......;p
See the H-P-H bond angle given there as 93.5 degrees
This is anoth3r contradiction jux like H2S. The truth is that the cambrigde is afraid of facing the reality and tends to teach its students what is easy to understand, even if it's against the reality. Lemme elaborate wid a good example:- When we are young, we are told by our elders that the Earth is ROUND but when we grow older we find that actually it ain't ROUND, rather it's SPHERICAL.......;p