does that helped?Wa eyyakum.
Thank you again, but very sorry, I am confused in one thing,
where did you get 6 + 1 + 1??
JazakAllah khiaran, May Allah S.W.T reward you for all your help, Aameen.
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry and Physics some Keypoints/Notepoints (onDemandOnly) !! :)
- Thread starter xhizors
- Start date
- Messages
- 2,865
- Reaction score
- 13,723
- Points
- 523
F=Le
L=Avagadro no = 6.02x10^23
e=Charge ~ 1.6x10^-19
u get approx 96500 C/mol i guess
Jazakum Allahu Khair!see u just need to know the formula F = Le where F is the farady constant, L is avogadro's no and e is the charge of one electron. it is used to prove avogadro's no thats it...
But what Brother iKhaled stated is precisely the problem i face... ummm.. how shall i phrase this- alright:
What i mean to say is, yep, i get that there's the Farady's constant, the charge on one e- but what i don't get is the question 34 on page 295:/
I don't know how to prove this equation leads to the value, 6.02 x 10^23 .... so i would appreciate it if u could provide me with an explanation
Thank you very much!
Inshallah InshallahOoooh, you meant by 6 + 1 + 1 the 8 splits. I thought something else. P.S :Not awesome protons, headache!
Anyway, I now got all what you have said, JazakAllah khairan. Thanks alot!
May Allah S.W.T help and guide you and your family to follow the steps of our beloved Prophet allahi assalatu wa salam. And In Sha Allah, you will be granted with the world distinction.
May Allah grant you double of what you have prayed for me!
Ameen.
- Messages
- 2,865
- Reaction score
- 13,723
- Points
- 523
me 2Inshallah Inshallah
May Allah grant you double of what you have prayed for me!
Ameen.
- Messages
- 844
- Reaction score
- 2,495
- Points
- 253
yup!
In that case shouldn't it be 7? total number of protons in the adjacent carbons = 6
so peak split = 6+ 1 = 7
We use the n+1 rule after counting the total number of adjacent proton and not protons on each adjacent carbon.
Ameen Ameenme 2
- Messages
- 55
- Reaction score
- 5
- Points
- 18
Bismillah,
MashaAllah a BIG question
Ok so i assume you know about Peaks
we can deduce the structure by high resolution NMR where the peaks are splits apart
Peaks Splitting Depends on(highResolution NMR):
the proton environment in the neighbour (lol not in my neighbour)
the one which carbon is attatched to specifically
the pattern of splitting is: number of H atoms in neighbour +1
so assume fragment of CH3 is detected and it is attatched to Ch2 and on other side with ch2
so the split is breaking in 6let(6peaks)
Condition:
if Ch3 is attatched to most electronegative environment like Ch3-OH
then ch3 peak wont be splitting
ok so quest:
if its like ch3-ch2-OH how many peaks if so and how'll they split
now by neighbourhood u can get an idea of Hydrogens etc
Nmr Peaks x-axis is Chemical Shifts Values
so after judging what could possibly be around from peaks u pick a data booklet and match your prediction by Chemical shift values
Hope you get the idea!
now tell me where you have confusion
can't we split ch2 with 3 peaks first ? and then show other splitting pattern for the second in the formulae ch2? is it necessary to show altogether 6 peaks?
and why are we doubling the height as in the LOW resolution NMR?
letme confirm btw thanks!In that case shouldn't it be 7? total number of protons in the adjacent carbons = 6
so peak split = 6+ 1 = 7
We use the n+1 rule after counting the total number of adjacent proton and not protons on each adjacent carbon.
- Messages
- 55
- Reaction score
- 5
- Points
- 18
second ch2* in the formulae?
- Messages
- 869
- Reaction score
- 374
- Points
- 73
whaaaat?!! come onnnnn from what i said above u should be able to do this question with closed eyes just use the formula F = LeJazakum Allahu Khair!
But what Brother iKhaled stated is precisely the problem i face... ummm.. how shall i phrase this- alright:
What i mean to say is, yep, i get that there's the Farady's constant, the charge on one e- but what i don't get is the question 34 on page 295:/
I don't know how to prove this equation leads to the value, 6.02 x 10^23 .... so i would appreciate it if u could provide me with an explanation
Thank you very much!
F = 96 485 and e = 1.6022 x 10^-19
L = 96 485/(1.6022 x 10^-19)
L = 6.0220 x 10^23
PROVED!! got it now ?
- Messages
- 55
- Reaction score
- 5
- Points
- 18
can someone explain me the mechanism of nucleophilic addition of propanal with HCN?
- Messages
- 2,865
- Reaction score
- 13,723
- Points
- 523
whaaaat?!! come onnnnn from what i said above u should be able to do this question with closed eyes just use the formula F = Le
F = 96 485 and e = 1.6022 x 10^-19
L = 96 485/(1.6022 x 10^-19)
L = 6.0220 x 10^23
PROVED!! got it now ?
ummm...thanks..JazakAllah.....
- Messages
- 869
- Reaction score
- 374
- Points
- 73
Yes Sir! Got it.....
ummm...thanks..JazakAllah.....
http://www.chemguide.co.uk/mechanisms/nucadd/hcn.htmlcan someone explain me the mechanism of nucleophilic addition of propanal with HCN?
http://www.chemguide.co.uk/mechanisms/nucadd/hcntt.html#top
- Messages
- 2,865
- Reaction score
- 13,723
- Points
- 523
Bismillahir RahmanirRahim...can someone explain me the mechanism of nucleophilic addition of propanal with HCN?
you see the oxygen atom has very high electronegativity yeah? so it leaves the C with a partial positive charge by pulling away it's e- or drawing them away from the C atom; the C atom is therefore prone to attack by the CN- ion. (the Cn- ion has a lone pair
This froms a negatively charged intermediate which is very reactive and immediatly reacts with the H+ ion (from the HCN).
and viola! a hydroxynitrile is formed
U want me to upload the mechanism with the curly arrows and all?
- Messages
- 2,865
- Reaction score
- 13,723
- Points
- 523
- Messages
- 55
- Reaction score
- 5
- Points
- 18
thank you so much.. it really helped
- Messages
- 869
- Reaction score
- 374
- Points
- 73
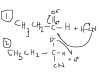
here i attached the mechanism but as always excuse my shity drawingscan someone explain me the mechanism of nucleophilic addition of propanal with HCN?
see there is a dipole on the carbon and oxygen in the c=o bond as u can see..the lone pair in the CN will attach the positive dipole on carbon and will form a C-CN bond this will leave a negative charge on the oxygen which will attract the positive H and BOOM u will have ur new hydroxy compound!
AGAIN MY DRAWINGS IS SHIIIIT..I HOPE U GET IT :$
Attachments
- Messages
- 55
- Reaction score
- 5
- Points
- 18
no i actually got it .. thanks btwBismillahir RahmanirRahim...
you see the oxygen atom has very high electronegativity yeah? so it leaves the C with a partial positive charge by pulling away it's e- or drawing them away from the C atom; the C atom is therefore prone to attack by the CN- ion. (the Cn- ion has a lone pair)
This froms a negatively charged intermediate which is very reactive and immediatly reacts with the H+ ion (from the HCN).
and viola! a hydroxynitrile is formed
U want me to upload the mechanism with the curly arrows and all?
- Messages
- 2,865
- Reaction score
- 13,723
- Points
- 523
LOL!here i attached the mechanism but as always excuse my shity drawings
see there is a dipole on the carbon and oxygen in the c=o bond as u can see..the lone pair in the CN will attach the positive dipole on carbon and will form a C-CN bond this will leave a negative charge on the oxygen which will attract the positive H and BOOM u will have ur new hydroxy compound!
AGAIN MY DRAWINGS IS SHIIIIT..I HOPE U GET IT :$
I expected "shit" if u drew it on paint