http://www.xtremepapers.com/papers/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s08_qp_4.pdf
can someone please explain QUES 8 b?
can someone please explain QUES 8 b?
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Well nw we have to link up all the tri peptides in such a way that we get the whole polypeptide .....this is done by trial and error methodhttp://www.xtremepapers.com/papers/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s08_qp_4.pdf
can someone please explain QUES 8 b?
oh i get it now! thankyouWell nw we have to link up all the tri peptides in such a way that we get the whole polypeptide .....this is done by trial and error method
The first aminoacid has to be methionine and the last aminoacid should be lysine
So the order will be met-ala-gly-all-gly-arg-val -lys
Hi just wanna ask what does "all"representThanks.Well nw we have to link up all the tri peptides in such a way that we get the whole polypeptide .....this is done by trial and error method
The first aminoacid has to be methionine and the last aminoacid should be lysine
So the order will be met-ala-gly-all-gly-arg-val -lys
Oops sorry ....thats was a spelling mistake :/ This was meant to be "ala" for the aminoacid AlanineHi just wanna ask what does "all"representThanks.
I think the answer is wrong because the e reduction-e oxidation=1.36-0.77=>+ve value,suppose to be feasible.Assalamu Alaikum there
M/j 2002 Q4 part c)i) y there is no reaction ?
http://freeexampapers.com/index.php/directory/download?location=A Level/Chemistry/CIE/2002 Jun/9701_s02_ms_4.pdf
Hi guys.Can anyone mind explaining this to me .Thanks guys.Cant understand even by refering to books.
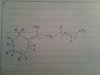
Thank u a lot.View attachment 39683
ALKENES REACTION .....We know under hot conc KMnO4 forms some some specific products which relies upon the functional carbon .....where it could primary ,secondary or tertiary carbon .....
So primary carbon always form carbondioxide and water ........
And secondary carbon always form carboxylic acid ......
And tertiary carbon always form for ketone ....
I hope u understand the pic
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now