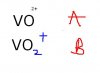yeah you will never find any thing wrong but it will never contain every single information you needSo i shouldn't read E.N. Ramsdan because there are errors in it...???
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 958
- Reaction score
- 3,499
- Points
- 253
Aoa wr wb Please do help me with my previously posted qn as well as the one that follows after.

The thing i don't get is why are we taking the lost water molecules to be 3, coz when 3 amino acids link, only 2 water molecules are released, not 3, as per my understanding......
This is an extract from the ms for this qn.

Can someone please help me with this qn
View attachment 19005View attachment 19006
i need a detailed explanation please, for both parts i and ii)
tx a million.
The thing i don't get is why are we taking the lost water molecules to be 3, coz when 3 amino acids link, only 2 water molecules are released, not 3, as per my understanding......
This is an extract from the ms for this qn.
- Messages
- 887
- Reaction score
- 466
- Points
- 73
iridium has two isotopes of mass numbers 191 and 193 and its average relative atomic mass is 192.23. calculate the relative abundances of the two isotopes... can anyone solve it the answers are 61.5%for 193 and 38.5% for 191 well i did get these answers via substitution can anyone solve it by any other means
- Messages
- 958
- Reaction score
- 3,499
- Points
- 253
Reposting my questions. Urgent help needed.......
Aoa wr wb Please do help me with these qns
Silent Hunter
Amy Bloom
I don't know anyone else doing A2, but if you are please do urgently helppp. thanx.
Aoa wr wb Please do help me with these qns
Can someone please help me with this qn
View attachment 19005View attachment 19006
i need a detailed explanation please, for both parts i and ii)
tx a million.
View attachment 19022
View attachment 19023
The thing i don't get is why are we taking the lost water molecules to be 3, coz when 3 amino acids link, only 2 water molecules are released, not 3, as per my understanding......
This is an extract from the ms for this qn.
View attachment 19027
Silent Hunter
Amy Bloom
I don't know anyone else doing A2, but if you are please do urgently helppp. thanx.
- Messages
- 1,857
- Reaction score
- 2,374
- Points
- 273
Hi. Well i'm unsure for part (ii) Confirm from someone else please. hope i helped.Reposting my questions. Urgent help needed.......
Aoa wr wb Please do help me with these qns
Silent Hunter
Amy Bloom
I don't know anyone else doing A2, but if you are please do urgently helppp. thanx.
I'll try to solve the other questions tomorrow if u don't mind.

- Messages
- 958
- Reaction score
- 3,499
- Points
- 253
Hi. Well i'm unsure for part (ii) Confirm from someone else please. hope i helped.
I'll try to solve the other questions tomorrow if u don't mind.
View attachment 19056
heya tx a million sis, um for part i) though, the answer is G>E>F and part ii) only G reacts with Na2CO3 to produce effervescence
and with NaOH E and G both dissolve.
i kinda reasoned out stuff the same way you did above, but as i mentioned above the ms states otherwise:/
and about the second qn, my exam is tomorrow
tx again
- Messages
- 1,857
- Reaction score
- 2,374
- Points
- 273
Hi soldier!heya tx a million sis, um for part i) though, the answer is G>E>F and part ii) only G reacts with Na2CO3 to produce effervescence
and with NaOH E and G both dissolve.
i kinda reasoned out stuff the same way you did above, but as i mentioned above the ms states otherwise:/
and about the second qn, my exam is tomorrowdon't want to bother you, but if it's possible and you can do it today, i'd be really really grateful
tx again
I'm so sorry i couldn't reply you on time because yesterday i had to rush. Still do u need the solutions?
Still if u need help, i'm there!
- Messages
- 105
- Reaction score
- 28
- Points
- 38
Can u plz answer...what is meant by ' high octane rating' in organic chemistry?
- Messages
- 958
- Reaction score
- 3,499
- Points
- 253
My test is over but yeah i'll definitely tell you if i do need help againHi soldier!
I'm so sorry i couldn't reply you on time because yesterday i had to rush. Still do u need the solutions?
Still if u need help, i'm there!
- Messages
- 49
- Reaction score
- 1
- Points
- 18
can anyone ans this for me....?
1)explain why hydrogen is evolved more slowly from methanoic acid solution than a HCL solution?
2)explain why, eventually,the methanoic acid sol produces as much hydrogen as the HCL solution?
add data given: Methanoic acid and hcl both are being reacted to an excess of Magnesium powder .
For a fixed amount of Mg, the rate equation for the reaction is:
rate= k [H+(aq)]
the paper:http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w03_qp_4.pdf
question is 2
1)explain why hydrogen is evolved more slowly from methanoic acid solution than a HCL solution?
2)explain why, eventually,the methanoic acid sol produces as much hydrogen as the HCL solution?
add data given: Methanoic acid and hcl both are being reacted to an excess of Magnesium powder .
For a fixed amount of Mg, the rate equation for the reaction is:
rate= k [H+(aq)]
the paper:http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w03_qp_4.pdf
question is 2
- Messages
- 57
- Reaction score
- 12
- Points
- 18
I have a question from May June 2008 PAPER 5 Q2 part f, i don't get how to draw these lines do we need to find the value and which x and y values to take? can someone plz explain.
f)Draw construction lines on the graph to derive values to enable you to calculate
the relative formula mass of the basic copper(II) carbonate and the value of x in
CuCO3.Cu(OH)2.xH2O.
Values read from graph, including units
x-axis ...................................................... y-axis ...........................................................
Calculation of the relative formula mass, Mr
[Ar: H, 1.0; C, 12.0; O, 16.0; Cu, 63.5]
f)Draw construction lines on the graph to derive values to enable you to calculate
the relative formula mass of the basic copper(II) carbonate and the value of x in
CuCO3.Cu(OH)2.xH2O.
Values read from graph, including units
x-axis ...................................................... y-axis ...........................................................
Calculation of the relative formula mass, Mr
[Ar: H, 1.0; C, 12.0; O, 16.0; Cu, 63.5]
- Messages
- 38
- Reaction score
- 17
- Points
- 18
Methanol is much more toxic than ethanol, what does the toxicity of alcohols depend on ??? Does it depend on, how long the carbon chain is ???
- Messages
- 639
- Reaction score
- 842
- Points
- 103
Possibly, since the length of the chain affects what end products are produced. Ethanol and Methanol both compete for the enzyme 'Alcohol dehdryogenase' in the body, methanol forms formaldehyde whereas ethanol form acetaldhyde. Formaldehdy is poisonous implying that methanol is more toxic.
- Messages
- 460
- Reaction score
- 293
- Points
- 73
Balance this equation using the oxidation number method?
V3+ + I2 + H2O >>> VO2+ + I- + H+
I tried using the method but it kept stumping me, there's one step I seem to be getting wrong. I'd appreciate if someone were to show step by step how to balance it using the oxidation number method,
Thanks!
V3+ + I2 + H2O >>> VO2+ + I- + H+
I tried using the method but it kept stumping me, there's one step I seem to be getting wrong. I'd appreciate if someone were to show step by step how to balance it using the oxidation number method,
Thanks!
- Messages
- 1,857
- Reaction score
- 2,374
- Points
- 273
Hi what's the answer, so that i can check if i'm right.Balance this equation using the oxidation number method?
V3+ + I2 + H2O >>> VO2+ + I- + H+
I tried using the method but it kept stumping me, there's one step I seem to be getting wrong. I'd appreciate if someone were to show step by step how to balance it using the oxidation number method,
Thanks!
- Messages
- 460
- Reaction score
- 293
- Points
- 73
It's 2V3+ + I2 + 2H2O >>> 2VO2+ + 2I- + 4H+Hi what's the answer, so that i can check if i'm right.
- Messages
- 460
- Reaction score
- 293
- Points
- 73
Oh sorry for the confusion it's A not BCan u confirm this A or B?View attachment 19154
- Messages
- 460
- Reaction score
- 293
- Points
- 73
I got most of it but, why did you put a 2 next to H2O and how is it an oxidising agent?Hi aalmuhannadi. sorry it took me time to put up explanations. I hope u'll understand in this mess.
Humm... i have put sme indications, follow the numbers.
View attachment 19160
Somebody else check this please.