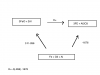
Please help with the following questions:Please help with the following questions:
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s11_qp_12.pdf
Q23 Q36
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s12_qp_11.pdf
Q13 Q22
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w12_qp_13.pdf Q10
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s11_qp_12.pdf
Q23 Q36
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s12_qp_11.pdf
Q13 Q22
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w12_qp_13.pdf Q10
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s12_qp_12.pdf Q 20