- Messages
- 616
- Reaction score
- 2,961
- Points
- 253
Whats thatTwo words: Steric Hindrance!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Whats thatTwo words: Steric Hindrance!
why would we add cl in double bonds?Isnt the salt in second one CaCl as they have referred to reaction 1 and formation of CaCl is reaction 1
Also in the last one why dont we add Cl in double bonds?
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w10_qp_23.pdf in Q3 a-ii) how do we know what is the shape?
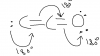
in simple words atoms take some space. so for them to bond there must be enough space. fluorine is very small so not more than one atom can bond with. as the two atoms will have to be very close to each other to bond with fluorine at same time.Whats that??
Thunkooo ^_^in simple words atoms take some space. so for them to bond there must be enough space. fluorine is very small so not more than one atom can bond with. as the two atoms will have to be very close to each other to bond with fluorine at same time.
other explanation might be that because the Fluorine atom is very small because the atom outer shell is very small. so not it cannot allow two more electrons in it. the repulsion of electrons will be very large.
A levels has a percentile system. the threshold is decided ever year separately accordingly to the resultscan anyone tell me what the limits of the threshold are? (minimum and maximum mark required to get an A) in as level
Wrong! Everything in every science is fully logic based. We, students, tend to ignore the logic in finding shortcuts/ways things are easy for US to remember/explain.
You stand up for the chemists Abby!
normally we are required to write in 3 s.f. unless otherwise stated in the question.Can someone tell me how do we know how many significant figures v have to use in chem and other subjects(bio, phy)
Is this rule applicable that you write ans in max sig fig mentioned in some value given in question?normally we are required to write in 3 s.f. unless otherwise stated in the question.
Thankswhy would we add cl in double bonds?
Cl will add in double bonds as result of electrophilic addition. whereas the mechanism of substitution is very different.
there are two carbons and one oxygen. the carbons has four electrons available for bonding. oxygen has 2 electrons available for bonding. the sequence is Carbon-Carbon-Oxygen
so carbon C=C=O will be most feasible structure.
View attachment 40495
as the chain is straight the shape is linear.
Thanks
Also
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s11_qp_21.pdf
Question 3 d)
Isnt the salt in this part CaCl2 as they have referred to first reaction and that is formation of calcium chloride
Abbby, or anyone...
Could someone explain me (in enough detail so I understand, rather than memorize) Q6f(ii) & (iii).
Thank you in advance!
This same question has been bothering me for a while. This and the weird boxes one in the organic flowchart sorta thing in variant 42 I believe. These ON13 papers at large have been very, very weird. I dearly hope this isn't the case in like a week. Difficult is okay, but this shit is a whole new level of twisted.
"Difficult is okay, but this shit is a whole new level of twisted." Hhahahahahhaa, for sure its nothing too bad, Its just not taught anywhere in the syllabus!!
yes it is.... especially when u have collected data and stuff like in prcticals or practical related questions.Is this rule applicable that you write ans in max sig fig mentioned in some value given in question?
No.The thing is we have to imagine some things.In Japan and many other developed countries explanations are done using special models and very advanced media.We(especially the South east Asians) have to imagine all the things.We dont exactly visualize what happens.But still its true that South east asians score better than rest of the worldWrong! Everything in every science is fully logic based. We, students, tend to ignore the logic in finding shortcuts/ways things are easy for US to remember/explain.
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now