- Messages
- 253
- Reaction score
- 1,195
- Points
- 153
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s10_qp_42.pdf
can any1 explain 3b plz?
can any1 explain 3b plz?
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Problem is should we show the workings step by step because in some papers there are questions to calculate the moles and then the concentration in same part.

Its Better If you Show Ur working and Be on Safe SideProblem is should we show the workings step by step because in some papers there are questions to calculate the moles and then the concentration in same part.
If we want we can simplify and write it down in one step like the marking scheme does. But what should we do actually simplify or write it step wise?
And another question is H2O2 a oxidising agent or a reducing agent and why?
One more, is silver sulfate a prercipitate? Because when doing a paper i thought that if their is a sulfate ion if we test that for Cl-(although it doesn't have) we might get a white precipitate if silver sulfate is ppt. and that might mislead us to thinking it has a Cl- ion.
But if You read the question carefully it says Write the Formula of ORGANIC Product.. so CO2 is Not ORGANICFirst tell me that can I right Co2 instead of Carboxylic Acid when Alkene undergoes reaction with KMNO4 hot concentrated. because like the end product of hot Kmno4 is always co2 gas
Secondly.. how B and C are chiral ? (written in Ms) like they dont have different functional group attached to it.
View attachment 40722
9701 paper 23 May june 2012.
Suchal Riaz
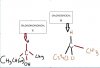
Thanks.Its Better If you Show Ur working and Be on Safe Side..
And H2O2 is Oxidizing Agent because H2O2 itself is reduced... while reacting it loses Oxygen to form water which is reduction.
eg . 2H+(aq) + H2O2(aq)+2Fe2+(aq) -> 2Fe3+(aq) +2H2O(l)
And yes Silver sulphate is insoluble . but i dont get your question?!
Oh! mistake.But if You read the question carefully it says Write the Formula of ORGANIC Product.. so CO2 is Not ORGANIC
Plz look at attachments.. (I m Sorry For Bad Drawing )
1-View attachment 40694
2-View attachment 40695
3-View attachment 40696
4-View attachment 40697
Anybody help me in this.
Suchal Riaz
I Could'nt Understand Frst 2 Attachements.. It would be better if u Post year and Question!
Much Better Question this Time...Thanks.
So basically your are saying to write all the working step by step without joining them into one step huh?
OK, the other question is say there is a a salt having both a Cl- and a sulfate ion, how do we confirm it has a Cl- ion?
Because if we test with AgNO3 we will anyway get a precipitate with both the 2 anions.
no year on themI Could'nt Understand Frst 2 Attachements.. It would be better if u Post year and Question!
Hydrogen Atoms Would only be H...can someone explain to me the difference between calculating the amount ,in moles, of hydrogen atoms and hydrogen molecules?
So Which two compounds Should we compare for Boilling points?no year on them
you think i dont know that?Hydrogen Atoms Would only be H...
Hydrogen Molecule Would Be H2!!
Haha..you think i dont know that?i meant difference in calculating them
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now