- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Out of every 100 g of fertilizer, 30 g is P205.
Out of 30 g of P2O5 ,
mass of P
= (2 x Mr of P/Mr of P2O5) x 30 g
= (62/142) x 30 g
=13.1 g
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
ThanksOut of every 100 g of fertilizer, 30 g is P205.
Out of 30 g of P2O5 ,
mass of P
= (2 x Mr of P/Mr of P2O5) x 30 g
= (62/142) x 30 g
=13.1 g
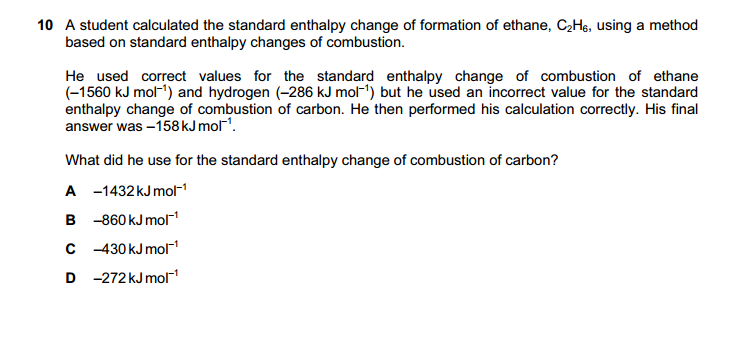
You haveHelp please, how do we solve this? Answer is C


thank you I thought it was the same atom that was broken..I2 (solid) --> I2 (gas)
The bond broken is from one I2 molecule to other I2 molecules, which is induced dipole (as I2 are non polar).
Covalent bonds are NOT broken as the molecules are not broken into atoms.
which exam is this?
Thank you so so much!!!
You didn't actually get my point though I needed explanation only on 11, 32, 33 and 35.
But anyway thanks so much for taking your time and explaining everything it might be of good use in the end.
Q1. Find out the number of moles of O2, since 1 mole of gas occupies 24000 cm^3 at RTP.
moles of O2 = 500/24 000
molecules of O2 = moles x 6.02 x 10^23 = (500/24000) x 6.02 x 10^23 = 1.25 x 10^22
Q2. Comparing the original structure to the resulting structure, we see that 5 C=C double bonds "gone".
So 5 moles of H2 are added.
If unable to visualise the above explanation, the diagram can show how H could be added in these locations.
View attachment 44133
Q7 and Q10
View attachment 44134
Q21. Is the answer really D? I got B.
View attachment 44136
Q22. The C=C undergoes oxidative cleaving, and the ends are oxidised to COOH.
View attachment 44137
Q23.
If confident, this question can be approached in a mathematical way
CnH(2n+2) + (3n+1)/2 O2 --> n CO2 + (n+1)H2O
when n increases, moles of O2 increases linearly. So its a line with a positive gradient.
Can you please help me with these as well

Answers are B and D
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s04_qp_1.pdf
Q9
Q35 isn't 3 suppose to be correct too?
Q37
Please anyone Q39. I cant understand a word.
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w08_qp_1.pdf
B is correct.
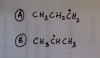
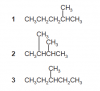
Can someone please help me in this question
May 2012 Paper 12 Q23
I would have definitely attached the link but I am from my mobile
Thanks in advance!!!
Thanks!!number of moles of Tl+NO3-=10*0.30/1000
=3*10^-3
Number of moles of NH4+VO3-=20*0.10/1000
=2*10-3
Divide by the smalles figure, we would obtain the ratio of 1.5:1 *2 to get rid of a fraction so the mole ration will 3:2 so for every 3 moles of Tl+( six electrons are removed) only two moles are reduced so divide the six electrons by 2 to figure out how many electrons are gained per mole
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now