- Messages
- 59
- Reaction score
- 130
- Points
- 43
There has to be a attachment to 2 ADJACENT carbons which is C
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
CView attachment 45117
full explanation plx..step by step
can u explain all the 3 ques plx
it takes no more than 1 minute. and you have 1.5 minutes to do each question.who i n helll will think to do this correct only 1 mark omg jesus christ the merciful
you could do that with H2 too.why isn't the number of moles of H2 taken into account? why only N2? :/ :S
Need helpView attachment 45119
oh okaayy thanks a lotyou could do that with H2 too.
number of moles of H2 used : number of moles of NH3 = 3:2
so (60 000 - 48 000):x=3:2
X=12 000*2/3=8000 moles = 136 Kg
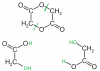
As the compounds reacts with itself to form the compound shown it must have both -OH and -COOH groups.oh okaayy thanks a lot
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s12_qp_11.pdf
Q23 ?? :/ (ANS - B)
I don't really get the compound C drawn :S
can yu also help me with this, since no one is answerin :/
Its the old rule of electrochem, the one in O levels.
question 9: the number of moles of solute increase on the right hand side so osmotic pressure increase
+1 to this,View attachment 45117
full explanation plx..step by step
can you just say what is confusing you in each question to make it easy for us to answer?http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w11_qp_12.pdf
Q37 (D)
Q26 (B)
Q9 (D)
Q29 (D)
Q35 (B)
Q40 (A)
P is endo, so 193Q4) Some bond energy values are listed below.
bond bond energy / kJ mol–1
C–H 410
C–Cl 340
Cl–Cl 244
Br–Br 193
These bond energy values relate to the following four reactions.
P Br2 → 2Br
Q 2Cl → Cl2
R CH3 + Cl → CH3Cl
S CH4 → CH3 + H
What is the order of enthalpy changes of these reactions from most negative to most positive?
A P → Q → R → S
B Q → R → S → P
C R → Q → P → S
D S → P → Q → R
calculate energy needed to break the bonds - energy released when bonds formed . the most negative asnwer on the right side and most positive to the left side. example:Q4) Some bond energy values are listed below.
bond bond energy / kJ mol–1
C–H 410
C–Cl 340
Cl–Cl 244
Br–Br 193
These bond energy values relate to the following four reactions.
P Br2 → 2Br
Q 2Cl → Cl2
R CH3 + Cl → CH3Cl
S CH4 → CH3 + H
What is the order of enthalpy changes of these reactions from most negative to most positive?
A P → Q → R → S
B Q → R → S → P
C R → Q → P → S
D S → P → Q → R
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w11_qp_12.pdf
Q37 (D)
Q26 (B)
Q9 (D)
Q29 (D)
Q35 (B)
Q40 (A)
Bro dont waste your time on already answered questionscalculate energy needed to break the bonds - energy released when bonds formed . the most negative asnwer on the right side and most positive to the left side. example:
in P: Br-Br is brocken and no bond is made so 193-0=+193
in Q: no bonds are broken only bond make is Cl-Cl so 0-244=- 244
in R CH3 + cl → CH3Cl only bond made is C-Cl. so -340
in S only bond broken is C-H = +410
negtive →→→ postive
-340(R) → -244(Q) → +193 → 410(S)
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now