- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Hmm, I'm suprised. Do you have the year of the paper?
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
I know it HAS to be AldehydeWell it seems, someoene already answered...
The Chill Master : you gotta check my reasoning..bcos in question 8 i think the functional group is Ketone and Aldehyde and not alcohol..Bcos according to my understanding if its alcohol the valency of carbon is not filled...draw the displayed formula and see if i make sense
http://postimg.org/image/8jdjxeqi1/
And I'm confused.Hmm, I'm suprised. Do you have the year of the paper?
Oh, Cl2 is reduced to HCl. Oxidation state of Cl from 0 to -1And I'm confused.
At the moment I don't but will find it soon.
(You are asking me right?)
Oh, Cl2 is reduced to HCl. Oxidation state of Cl from 0 to -1And I'm confused.
At the moment I don't but will find it soon.
(You are asking me right?)
So the other one is oxidised and it becomes redox....so 2 is redoxOh, Cl2 is reduced to HCl. Oxidation state of Cl from 0 to -1
Yes , the carbon in methane is oxidised, -4 to -3So the other one is oxidised and it becomes redox....so 2 is redox
and so is 1 as you mentioned earlier
the answer is B ( 1 and 2)
OkaayYes , the carbon in methane is oxidised, -4 to -3
Don't forget that the complete definition also includes a portion where the partially positive H atom is attracted to a lone pair of a neighboring molecule.
The CO2 is responsible for the yellow portion.
http://www.answers.com/Q/Does_carbon_dioxide_form_hydrogen_bonds_with_water
can somebody solve thissView attachment 51422
Not really, remember that organic molecules also have a hydrophobic segment. Recall why alcohols become less soluble as the chain increases. H bonding needs to be significant enough to allow a molecule to dissolve in water, it doesn't mean that a substance will definitely dissolve just because it has H bonding,if that is the case then carbonyl compounds should also be soluble and so should be any ester! :|
H2O CO H2 CO2
initial no. of moles: 1 1 0 0
n at equilibrium : 1-x 1-x x x
* x = no. of moles of H2O and CO reacted
* whatever the position of equilibrium, there are 2 moles of gas present.
so, no. of H2 formed = 33.3/100 * 2 = 0.666
n(H2) = n(CO2) = 0.666
n(H2O) = n(CO) = 1 - 0.666 = 0.334
Therefore, Kc = (0.666^2)/(0.334^2)
= 3.97608 = 3.98
is the answer correct?
but an aldehydes means carbon aTom attached directly to functional group and and an h .Though I am not sureWell it seems, someoene already answered...
The Chill Master : you gotta check my reasoning..bcos in question 8 i think the functional group is Ketone and Aldehyde and not alcohol..Bcos according to my understanding if its alcohol the valency of carbon is not filled...draw the displayed formula and see if i make sense
http://postimg.org/image/8jdjxeqi1/
If the answer is A then it can be Ketone and alcohol because Alcohol will react with alcohol and not with ketone.I know it HAS to be Aldehydethe answer for that is A!
17 is A Bravo THNX
for question 25 1 AND 3 is not an option remember?
yes ...ALDEHYDEbut an aldehydes means carbon aTom attached directly to functional group and and an h .Though I am not sure
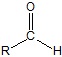
How did you got 17?Well it seems, someoene already answered...
The Chill Master : you gotta check my reasoning..bcos in question 8 i think the functional group is Ketone and Aldehyde and not alcohol..Bcos according to my understanding if its alcohol the valency of carbon is not filled...draw the displayed formula and see if i make sense
http://postimg.org/image/8jdjxeqi1/
Yeah and in that qstn this grp isn't present.yes ...ALDEHYDE


For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now