-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Yes, hot concentrated KMnO4 can oxidise alcohols.Guys in june 13 v23 5 a iv how did we get a dioic? does KMnO4 oxidise primary alcohols to acids?
However, cold one cannot.
Also note that K2Cr2O7 cannot break a double bond.
Thanks man.Yes, hot concentrated KMnO4 can oxidise alcohols.
However, cold one cannot.
Also note that K2Cr2O7 cannot break a double bond.
No, CH3CO2(*C*H3)CH3 this carbon will be bonded to 5 that way, 3 hydrogen atoms, 1 oxygen atom, 1 carbon atom.http://freeexampapers.com/A-Level/Chemistry/CIE/2010-Jun/9701_s10_qp_22.pdf
For Question 4 part c can one of the esters of C4H8O2 he CH3CO2(CH3)CH3? please someone help!??
basic hydrolysis giving ethanoate salt and alcoholReaaction bw CH3CO2CH(CH30)2 with KOH(aq) warm? What type of reacton is this and whats the product?
- Messages
- 424
- Reaction score
- 145
- Points
- 53
- Messages
- 1
- Reaction score
- 0
- Points
- 1
- Messages
- 187
- Reaction score
- 191
- Points
- 53
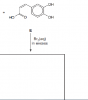
Hello Guys can you please help me: when there is a phenol group on the ring and you add Br2 (Aq) substitution occurs on 2,4,6 carbons. Many questions came where there are two phenol groups on the ring how will I know on which position bromine is added?
example is addedView attachment 53048
I believe that the bromine atoms would attach themselves across the double bond. So I'd basically draw that same structure, without the double bond, and a Br atom attached to each carbon that was present in the double bond.
- Messages
- 20
- Reaction score
- 2
- Points
- 13
When Magnesium burns (in Oxygen/Steam) It burns with a white flame. For the observation of the reaction, should I use this or should I say Magnesium glows?? Because books mention how Magnesium burns with a white flame while Marking scheme says Magnesium glows??
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Magnesium burns with a sparkling bright flame. Better not to mention any colour for it, we don't consider white a colour.When Magnesium burns (in Oxygen/Steam) It burns with a white flame. For the observation of the reaction, should I use this or should I say Magnesium glows?? Because books mention how Magnesium burns with a white flame while Marking scheme says Magnesium glows??
Sodium burns with golden yellow flame.
Sulfur burns with a pale blue flame.
I think phosphorus burns with yellow flame not sure though.
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Hello Guys can you please help me: when there is a phenol group on the ring and you add Br2 (Aq) substitution occurs on 2,4,6 carbons. Many questions came where there are two phenol groups on the ring how will I know on which position bromine is added?
example is addedView attachment 53048
On the benzene ring, you can add 2 bromine atoms. Next to the OH groups.
- Messages
- 1,318
- Reaction score
- 1,374
- Points
- 173
Anyone have a table of some sort or anything with all the reactions, reagents and conditions needed to be know for AS chemistry?
This would be for organic...
Please!
This would be for organic...
Please!
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
On the benzene ring, you can add 2 bromine atoms. Next to the OH groups.
Best organic notes please?Anyone have a table of some sort or anything with all the reactions, reagents and conditions needed to be know for AS chemistry?
This would be for organic...
Please!
mehria
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
- Messages
- 1,318
- Reaction score
- 1,374
- Points
- 173
The hydrogens are attached to the carbonsfor anything to be soluble in water, hydrogen bonds should exist between water and that substance right??
but in this ques MS says
hydrogen bonding is absent between Ethoxyethane moleculeswhy?
For hydrogen bonding, you need a H attached to a F,O or N
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Firstly, not all soluble substances have hydrogen bonding. Does NaCl have hydrogen bonds? No. (this dissolves because they are ions)for anything to be soluble in water, hydrogen bonds should exist between water and that substance right??
but in this ques MS says
hydrogen bonding is absent between Ethoxyethane moleculeswhy?
Secondly, in this question it says they form two layers. So ethoxyethane or whatever it is did NOT dissolve in water.
Thirdly, the molecule they gave cannot possibly have hydrogen bonds, it only has permanent dipole dipole interaction.
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
a
in ans to this I wrote because Hydrogen bonds donot form between ethoxyethane and H2O
but the Ms says
hydrogen bonds cannot form btween Ethoxyethane molecules :/
I think you didn't understand my questionFirstly, not all soluble substances have hydrogen bonding. Does NaCl have hydrogen bonds? No. (this dissolves because they are ions)
Secondly, in this question it says they form two layers. So ethoxyethane or whatever it is did NOT dissolve in water.
Thirdly, the molecule they gave cannot possibly have hydrogen bonds, it only has permanent dipole dipole interaction.
in ans to this I wrote because Hydrogen bonds donot form between ethoxyethane and H2O
but the Ms says
hydrogen bonds cannot form btween Ethoxyethane molecules :/
- Messages
- 27
- Reaction score
- 79
- Points
- 23
http://maxpapers.com/syllabus-mater...chment/9701_oct-nov-2011-all-question-papers/
Q4 W ... Q5 b and c anyone?
Q4 W ... Q5 b and c anyone?
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
paper?http://maxpapers.com/syllabus-mater...chment/9701_oct-nov-2011-all-question-papers/
Q4 W ... Q5 b and c anyone?
I have 5c paper 22 explanation of ma frn.
I will upload that.
Last edited:
- Messages
- 27
- Reaction score
- 79
- Points
- 23
Oh stupid me.. forgot to mention paper:paper?
November 11 9701/22