-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 2,266
- Reaction score
- 12,402
- Points
- 523
It means that different samples of the substance might have different melting points ... so basically it has a range of melting points ...View attachment 54828
What does it mean by it melts over a range of temperature?
- Messages
- 2,266
- Reaction score
- 12,402
- Points
- 523
is the answer A?
yes how?is the answer A?
- Messages
- 2,266
- Reaction score
- 12,402
- Points
- 523
It has the most number of C-Cl bonds which persist high up in the atmosphere and break to form chlorine free radicals that deplete the ozone layer ... C-H bonds will break sooner once released into the atmosphere ... and C-F bonds are stable so they dont break as easily.yes how?
- Messages
- 1,318
- Reaction score
- 1,374
- Points
- 173
Oh hahaha I didn't know polyethene did thatIt means that different samples of the substance might have different melting points ... so basically it has a range of melting points ...
thnksIt has the most number of C-Cl bonds which persist high up in the atmosphere and break to form chlorine free radicals that deplete the ozone layer ... C-H bonds will break sooner once released into the atmosphere ... and C-F bonds are stable so they dont break as easily.
- Messages
- 2,266
- Reaction score
- 12,402
- Points
- 523
different samples of polymers can have different no. of monomers to make it up ... the longer the chain, the greater the melting point .... thus the rangeOh hahaha I didn't know polyethene did that
no problemthnks
different samples of polymers can have different no. of monomers to make it up ... the longer the chain, the greater the melting point .... thus the range
no problem
Bro how to solve Oct nov 2014 Q 30 plz
http://maxpapers.com/syllabus-materials/chemistry-9701-a-level/attachment/9701_w14_qp_11/
My answer is 8
- Messages
- 6
- Reaction score
- 5
- Points
- 3
- Messages
- 2,266
- Reaction score
- 12,402
- Points
- 523
Bro how to solve Oct nov 2014 Q 30 plz
http://maxpapers.com/syllabus-materials/chemistry-9701-a-level/attachment/9701_w14_qp_11/
My answer is 8though its not in the marking scheme
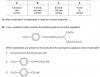
See, the molecule given has a lot of C=C bonds ... but only the tertiary alkenes will oxidise to give ketones ... (the secondary alkenes will oxidise to give aldehyde which further get oxidised to form acids. The primary ones give CO2) ... tertiary alkenes is when a C in the C=C double bond is attached to 3 other Carbon atoms ... if u look at the molecule carefully, u will see that there are 6 tertiary alkenes.
- Messages
- 2,266
- Reaction score
- 12,402
- Points
- 523
and oh! i am a girlSee, the molecule given has a lot of C=C bonds ... but only the tertiary alkenes will oxidise to give ketones ... (the secondary alkenes will oxidise to give aldehyde which further get oxidised to form acids. The primary ones give CO2) ... tertiary alkenes is when a C in the C=C double bond is attached to 3 other Carbon atoms ... if u look at the molecule carefully, u will see that there are 6 tertiary alkenes.
- Messages
- 6
- Reaction score
- 5
- Points
- 3
- Messages
- 2,266
- Reaction score
- 12,402
- Points
- 523
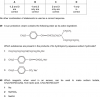
only the 39th question is in the picture ...Can You help me with number 39 & 40?View attachment 54830
Is the answer B? If so, then ...
alkaline (NaOH in this case) hydrolisis of ester has 2 products: the sodium salt of the acid and the alcohol ...
and the first part of the molecule where the C is double bonded to O becomes the salt of acid ... the other part becomes the alcohol.
ThanksSee, the molecule given has a lot of C=C bonds ... but only the tertiary alkenes will oxidise to give ketones ... (the secondary alkenes will oxidise to give aldehyde which further get oxidised to form acids. The primary ones give CO2) ... tertiary alkenes is when a C in the C=C double bond is attached to 3 other Carbon atoms ... if u look at the molecule carefully, u will see that there are 6 tertiary alkenes.
- Messages
- 2,266
- Reaction score
- 12,402
- Points
- 523
40th question ... is the answer D?
- Messages
- 1,318
- Reaction score
- 1,374
- Points
- 173
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
Reaction of aldehydes with HCN require Reflux OR it occurs at RTP?
Help Please!!!
Use of the Data Booklet is relevant to this question. Sir Humphrey Davy discovered boron, calcium, magnesium and sodium. Which of these elements has the second smallest atomic radius in its group and the third lowest first ionisation energy in its period?
A boron
B calcium
C magnesium
D sodium
The Ans is C
Use of the Data Booklet is relevant to this question. Sir Humphrey Davy discovered boron, calcium, magnesium and sodium. Which of these elements has the second smallest atomic radius in its group and the third lowest first ionisation energy in its period?
A boron
B calcium
C magnesium
D sodium
The Ans is C
- Messages
- 6
- Reaction score
- 5
- Points
- 3
I think it its C.40th question ... is the answer D?