ok what about average atomic mass?From where did u got a phrase like Avg. isotopic mass?
The colourless solution produces a grey precipitate of silver, or a silver mirror on the test tube.
and thanks for clearing that tollen's problem.
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
ok what about average atomic mass?From where did u got a phrase like Avg. isotopic mass?
The colourless solution produces a grey precipitate of silver, or a silver mirror on the test tube.
Sorry no idea ... I never really did many papers and when i did, i didn't focus on the pattern .... So i would like to know as well.No one knows this?? Metanoia awesomaholic101
Can u solve my doubts?Sorry no idea ... I never really did many papers and when i did, i didn't focus on the pattern .... So i would like to know as well.
The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundanceok what about average atomic mass?
and thanks for clearing that tollen's problem.
divided by 100? thanksThe average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance
According to me, it should be relative atomic mass that ur talking about.divided by 100? thanksso we write as is?
my teacher was saying definitions like these will come.. so i'm just making sure.
I'll try nowCan u solve my doubts?
When I solve recent years past papers that is from 2007 to 2015(summer) summer and witner both I use to score always above 50. Now when I look into old years like this paper I ended up at the score of just 46. I have so many doubts in this paper. If anyone can help me out solving, Q1(c)(d), Q2(c)(d), Q5(c), Q7(b)(c) in Q7(b) arent answer in ms for uses of esters? Please do it as soon as possible. Also, When I was solving papers I found that mostly moles thingy are in summer papers, in winter papers its mostly inorganic part and theory. Am I correct? Should I expect 2015 november paper to be easier with moles thingy??
Thank you,
Regards,
XPC member,
The Sarcastic Retard

How do we know which line denotes what atom?I believed you have gone through the ms already which I am posting below.
Which part of the answers do you need to clarify?
Q1
View attachment 57215
Q2
View attachment 57216
Q5
View attachment 57217
View attachment 57218
Q7
View attachment 57219
As for patterns in setting of questions, I don't really look at it. I seldom encourage "spotting of questions".
Can anyone explain to me why the potential of the electrode decreases as [Ag+] decreases?
1)b)ii)
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w05_qp_4.pdf
Shouldnt it increases because in Ag+ + e => Ag, decreasing Ag+ would shift equilibrium to LHS so increasing the Eo?
How do we know which line denotes what atom?
How do we calculate the atomic radii of argon? Some data must be given about it in the data booklet right?
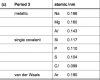
relative atomic mass isAccording to me, it should be relative atomic mass that ur talking about.
And yeah, definations are must.
Thank uHow do we know which line denotes what atom?
The useful background knowledge required is that chlorine has two main isotopes; Cl- 35 and Cl-37.
So, from left to right, we have Cl-35 , HCl (where the Cl is Cl-35), Cl-37 and HCl (where the Cl is Cl-37).
Even if we do not have that background knowledge, we should be able to deduced that the 4 lines are from
lightest Cl atom, HCl with lighter Cl atom, heavier Cl atom, HCl with heavier Cl atom
How do we calculate the atomic radii of argon?
This can be found in the data booklet. The numbers in the most recent edition might be different from the suggested value in the MS, keeping in mind that the paper was set more than 10 years ago.
View attachment 57220
Yeah, but he is asking about Avg atomic mass..relative atomic mass is
average mass of an atom of element relative to carbon 12. which is exactly 12 units. (ms)
Every thing Sir.I believed you have gone through the ms already which I am posting below.
Which part of the answers do you need to clarify?
Q1
View attachment 57215
Q2
View attachment 57216
Q5
View attachment 57217
View attachment 57218
Q7
View attachment 57219
As for patterns in setting of questions, I don't really look at it. I seldom encourage "spotting of questions".
I believe the doubts with the first question is solved.No one knows this?? Metanoia awesomaholic101
I personally find chemguide.co.uk very helpful for the organic part. Xtremepapers revision section is also available.Can some one share a link to topical papers for chemistry (a thread or a website) Appreciated.
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now