- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
Q30 : Are they correct?Qn 6.
Original Mr (CaCl2) = 111
Final Mr (CaCl2.2H2O) = 147
% Increased in Mr =(36/111) x 100% = 32%
Qn8.
Moles of Cr2O7 2- = 0.00131 mol
Moles of Fe2+ = 0.00131 x 6 = 0.00786 mol
Mass of Fe = 0.00786 x 56 = 0.44 g
% of Fe = (0.44/1) x 100% = 44%
Qn17.
Describing an element that forms an amphoteric oxide, so answer is Al.
Qn 18.
P4O10 + 6H2O --> 4H3PO4
Qn 19.
CO2 is released when HCl is added to CaCO3.
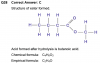
Qn30.
You can post your isomers, and I'll help to see what's missing.
Qn39.
Statement 1: true, hydrazone is orange
Statement 2: false, ethanoic acid is colorless
Statement 3: false, ethan-1,2- diol is colorless.
Qn40.
View attachment 57440

Q39 : statement 2 isnt color changes from orange to green. No?
Q40 : How statement 1 is correct?
THanks.