- Messages
- 140
- Reaction score
- 414
- Points
- 73
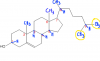
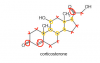
Those numbers indicate the chiral carbons right? But I am pretty sure number 9 is not a chiral carbon ... 1 to 8 are thoughhttp://www.chemguide.co.uk/basicorg/isomerism/cholesterol.gif
How do I identify the chiral carbon atom?
Ty in advance..