-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
Its Li3N + H2O not Li3N + O2In J11 p41
Li3N + 3H2O = xxxxx
I amswered it as lithium oxide, answer was lithium hydroxide.
why???
Li3N + 3H2O ----> 3Li(OH) + NH3
4Li3N + 3O2 ⇄ 6Li2O + 2N2
- Messages
- 340
- Reaction score
- 339
- Points
- 73
- Messages
- 340
- Reaction score
- 339
- Points
- 73
- Messages
- 340
- Reaction score
- 339
- Points
- 73
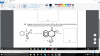
Positive test with acidified dichromate(VI) AND ALSO Tollen's reagent.View attachment 60170
Why is it X and Z only? when the OH in W can be oxidized to COOH and the carbonyl group, in the middle, will test positive with Tollen's!
Only Aldehydes will give positive test with Tollen's reagent.X and Z are the ones.
- Messages
- 340
- Reaction score
- 339
- Points
- 73
oh yes yes, Tollen's is a test for aldehydes! not for carbonyls! thanksPositive test with acidified dichromate(VI) AND ALSO Tollen's reagent.
Only Aldehydes will give positive test with Tollen's reagent.X and Z are the ones.
can u help with my other questions?
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Because it contains the C-I bond! It will break the quickest since C-I bond is very weak. So it forms ppt the fastest.View attachment 60171
It's D.
I thought A since Florine is the most reactive element, the C-F bond is strongest and most difficult to break, but it's D. How and why will it produce precipitate quickest?
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Well I'm not sure how you concluded that the amount of P at equilibrium would be 2 moles, but basically there's two ways of doing this:View attachment 60169
Moles of R at equilibrium are x, so P will have 2+x-x =2 what about Q?
(Ans B)
1. Trial and Error.
We will assume A to be answer:
Since R increased by x moles, we expect P to decrease by x and Q to increase by 2x.
In the 'Final' row, if you add up all the amounts, you get: (2-x) + 2x + x = 2+2x moles. This does not match with what the question specified. So we reject this.
Next we assume B to be the answer:
When you add these final moles up, the answer is: 2-2x + 2x + x = 2+x moles. This is as the question required, so our answer is B.
Questions where they expect candidates to do trial and error often have B as the answer, as they want to test your concept but they don't want to waste your time.
But let's say you don't like trial and error....
2. Algebra!
We will assume our equation to be of the following form:
Notice that we assume the coefficient of R to be 1. We can make this assumption about any one specie in a chemical reaction. If a and b turn out to be 1.5 and 2.5 for example, then we can multiply the whole equation through by 2 so that the coefficients of all species become whole numbers.
Anyhow, if you add the final moles in the above equation, you get:
2 - ax + bx + x = 2 + (b - a + 1)*x
The question said there should be 2 + 1*x moles, so we can equate coefficients of x:
2 + (b - a + 1)*x ≡ 2 + x
b - a + 1 = 1
b - a = 0
b = a
This means the coefficient of P and Q have to equal! If you look through the choices, only option B satisfies this requirement.
Which ones? I can try. :3oh yes yes, Tollen's is a test for aldehydes! not for carbonyls! thanks
can u help with my other questions?
( If you mean the next questions,they've been answered.)
- Messages
- 340
- Reaction score
- 339
- Points
- 73
oh my! thank you so much!!Well I'm not sure how you concluded that the amount of P at equilibrium would be 2 moles, but basically there's two ways of doing this:
1. Trial and Error.
We will assume A to be answer:
View attachment 60175
Since R increased by x moles, we expect P to decrease by x and Q to increase by 2x.
In the 'Final' row, if you add up all the amounts, you get: (2-x) + 2x + x = 2+2x moles. This does not match with what the question specified. So we reject this.
Next we assume B to be the answer:
View attachment 60176
When you add these final moles up, the answer is: 2-2x + 2x + x = 2+x moles. This is as the question required, so our answer is B.
Questions where they expect candidates to do trial and error often have B as the answer, as they want to test your concept but they don't want to waste your time.
But let's say you don't like trial and error....
2. Algebra!
We will assume our equation to be of the following form:
View attachment 60177
Notice that we assume the coefficient of R to be 1. We can make this assumption about any one specie in a chemical reaction. If a and b turn out to be 1.5 and 2.5 for example, then we can multiply the whole equation through by 2 so that the coefficients of all species become whole numbers.
Anyhow, if you add the final moles in the above equation, you get:
2 - ax + bx + x = 2 + (b - a + 1)*x
The question said there should be 2 + 1*x moles, so we can equate coefficients of x:
2 + (b - a + 1)*x ≡ 2 + x
b - a + 1 = 1
b - a = 0
b = a
This means the coefficient of P and Q have to equal! If you look through the choices, only option B satisfies this requirement.
- Messages
- 456
- Reaction score
- 280
- Points
- 73
Just wondering how long that took you to typeWell I'm not sure how you concluded that the amount of P at equilibrium would be 2 moles, but basically there's two ways of doing this:
1. Trial and Error.
We will assume A to be answer:
View attachment 60175
Since R increased by x moles, we expect P to decrease by x and Q to increase by 2x.
In the 'Final' row, if you add up all the amounts, you get: (2-x) + 2x + x = 2+2x moles. This does not match with what the question specified. So we reject this.
Next we assume B to be the answer:
View attachment 60176
When you add these final moles up, the answer is: 2-2x + 2x + x = 2+x moles. This is as the question required, so our answer is B.
Questions where they expect candidates to do trial and error often have B as the answer, as they want to test your concept but they don't want to waste your time.
But let's say you don't like trial and error....
2. Algebra!
We will assume our equation to be of the following form:
View attachment 60177
Notice that we assume the coefficient of R to be 1. We can make this assumption about any one specie in a chemical reaction. If a and b turn out to be 1.5 and 2.5 for example, then we can multiply the whole equation through by 2 so that the coefficients of all species become whole numbers.
Anyhow, if you add the final moles in the above equation, you get:
2 - ax + bx + x = 2 + (b - a + 1)*x
The question said there should be 2 + 1*x moles, so we can equate coefficients of x:
2 + (b - a + 1)*x ≡ 2 + x
b - a + 1 = 1
b - a = 0
b = a
This means the coefficient of P and Q have to equal! If you look through the choices, only option B satisfies this requirement.
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Well I didn't really check but perhaps 10 minutes? This one was slightly longer because I wanted to explain in detail since I knew last year others had this issue as well. This way next time someone asks this question again I can just link them to this answerJust wondering how long that took you to type

There have been so many questions asked and answered on this thread over the years that I think there should be some page where the answers are somehow organized in such a way that if, for example, I was having trouble with q6 of ON 2007 paper 1, I could quickly see if someone has previously answered it. Though it seems difficult
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
Well I didn't really check but perhaps 10 minutes? This one was slightly longer because I wanted to explain in detail since I knew last year others had this issue as well. This way next time someone asks this question again I can just link them to this answer
There have been so many questions asked and answered on this thread over the years that I think there should be some page where the answers are somehow organized in such a way that if, for example, I was having trouble with q6 of ON 2007 paper 1, I could quickly see if someone has previously answered it. Though it seems difficult
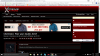
You can see this search button. For example I have doubt in 2007 november paper 4 I will search : 9701_w07_qp_4 and you will be having a new page, there u can press ctrl + f to search the question number for example I have doubt in Q2b(iii) so I will type 2 in the ctrl + f search space and then scroll down to see if there were any question asked previously. If not then post the doubt
Well I didn't really check but perhaps 10 minutes? This one was slightly longer because I wanted to explain in detail since I knew last year others had this issue as well. This way next time someone asks this question again I can just link them to this answer
There have been so many questions asked and answered on this thread over the years that I think there should be some page where the answers are somehow organized in such a way that if, for example, I was having trouble with q6 of ON 2007 paper 1, I could quickly see if someone has previously answered it. Though it seems difficult
Even I wish there was some page that could collect all the answers.Alot of us have the same doubt.The XPC search isn't that helpful always.
It doesn't always give all the possible pages.I've tried it.Googling is better as per my experience. :/View attachment 60180
You can see this search button. For example I have doubt in 2007 november paper 4 I will search : 9701_w07_qp_4 and you will be having a new page, there u can press ctrl + f to search the question number for example I have doubt in Q2b(iii) so I will type 2 in the ctrl + f search space and then scroll down to see if there were any question asked previously. If not then post the doubt
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
In that case, you can post the doubt with the link instead of pictures, then that will be easier for us to search..Thank you so much for all your help here.JazakAllah khair!
Even I wish there was some page that could collect all the answers.Alot of us have the same doubt.The XPC search isn't that helpful always.
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
For my As level the XPC search was pretty helpful. Google is also a good option.It doesn't always give all the possible pages.I've tried it.Googling is better as per my experience. :/