- Messages
- 73
- Reaction score
- 19
- Points
- 18
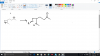
39 Okay, got that39)1 and 2
View attachment 61409
40) This is easiest last question, i ever saw xD Sorry to say like this, but just u have to manipulate its structure and match with the given compound in question. All three is having that formula after oxidation. Try once, if u dont get ask me. Bored in making that one in 39 xD
40 Yeah okay...but I don't get the 3rd molecule ? ANd hey Q 40 is in the examiner report's particularly difficult