- Messages
- 665
- Reaction score
- 13,607
- Points
- 503
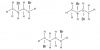
it gets oxidised to propanone by NaOH firsthttp://justpastpapers.com/cie-9701-...question-paper-and-mark-scheme/9701_m17_qp_12 in Q39 why would propan-2-0l give yellow ppt with iodine?
this propanone formed gives a positive test