-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 16
- Reaction score
- 2
- Points
- 3
there is no reaction possibly of acid with alkene. please check!
- Messages
- 2
- Reaction score
- 0
- Points
- 11
if u have 9701_m18_qp_42 plz share
- Messages
- 341
- Reaction score
- 224
- Points
- 53
- Messages
- 16
- Reaction score
- 2
- Points
- 3
here, from the spectra we can see there are 4 different carbon environment. so in isomer " R" there are 5 different carbon environment. while in isomer "S" there is only 3 carbon environment. as in isomer " T " there are four environment. hence it is spectra of isomer T
- Messages
- 15
- Reaction score
- 3
- Points
- 13
There is definitely a reaction with Hydrogen Halides, and they are also acids. HCl reacts with Alkenes in gaseous state or mixed in water as a concentrated solution, which is basically hydrochloric acid. This is because the hydrogen will act as a electrophile, as it already has a slightly positive charge due to the electronegative chloride ion.there is no reaction possibly of acid with alkene. please check!
- Messages
- 16
- Reaction score
- 2
- Points
- 3
sorry , i had misunderstood your question
- Messages
- 341
- Reaction score
- 224
- Points
- 53
Quite good, was easier than the last year.
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
View attachment 63545
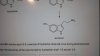
How do we come to know where to place the Cl and NH3? Cant there be any other arrangement apart from these?
View attachment 63546
There are only 2 unique ways to place the 2 Cl atoms, either 180 degrees from each other or 90 degrees
yeah its was, lets discuss what was the ph? for first question?Quite good, was easier than the last year.
- Messages
- 341
- Reaction score
- 224
- Points
- 53
1.6
- Messages
- 341
- Reaction score
- 224
- Points
- 53
Temperature was 2836 and volume 448cm^3
yes i have same temperature and volume but different ph. how did you get 1.6 mine was alot higherTemperature was 2836 and volume 448cm^3
- Messages
- 341
- Reaction score
- 224
- Points
- 53
after finding the moles you had to calculate the conc using n*1000/800 and then the ph formula
how did you find the concentration for the OH?Temperature was 2836 and volume 448cm^3
btw how many proton peaks with there be?after finding the moles you had to calculate the conc using n*1000/800 and then the ph formula