yes so there was ethandioc acid, 2 other products one of which was given..so why didnt we count carbon dioxide as carbon containing productthree carbon containing products
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 341
- Reaction score
- 224
- Points
- 53
actually ethandioc acid was further oxidized to CO2
- Messages
- 22
- Reaction score
- 3
- Points
- 13
Predictions for paper 41?
ohhhhhhhhhh rightttactually ethandioc acid was further oxidized to CO2
- Messages
- 24
- Reaction score
- 7
- Points
- 13
wasn't it for nitration of phenol?
It was about phenol
yes yes my bad wrote it in a hurryrate against time?? never heard of that ... It was Rate against concentration...
View attachment 63600
- Messages
- 85
- Reaction score
- 37
- Points
- 18
Btw, are october nov thresholds higher than may june ? I have seen this trend many times.. for papers of similar difficulty as well
- Messages
- 90
- Reaction score
- 14
- Points
- 18
H2+ICl= H2ICl then H2ICl + ICl = 2HCl +I2. Is this acceptable?Icl+h2=hcl+hi then hi+Icl= hcl+I2
- Messages
- 85
- Reaction score
- 37
- Points
- 18
what might the threshold be for chem.. varient 12,22,33,42,52.. do u guys think it will be above 205?
- Messages
- 86
- Reaction score
- 37
- Points
- 28
Btw, are october nov thresholds higher than may june ? I have seen this trend many times.. for papers of similar difficulty as well
Yes you are correct, I guess some People don't take mayjune seriously...and then retake In Oct NOv with full preparation...btw I think the Gt would be between 60-65 for P4
- Messages
- 196
- Reaction score
- 39
- Points
- 38
chemistry paper 41 anyone lmao
- Messages
- 33
- Reaction score
- 3
- Points
- 18
Yeah I did p41 today.. don’t ask for answers cuz I’m not sure if mine ..
- Messages
- 665
- Reaction score
- 13,607
- Points
- 503
well the reactant was waterwhat was the 2 mark equation for NH2COCH2CHNH2COOH and H2SO4?
but over the arrow we had to write H+
i think it acts as a catalyst thats y
- Messages
- 665
- Reaction score
- 13,607
- Points
- 503
well i think its shoulda been 2835Temperature was 2836 and volume 448cm^3
becuz rounding it off to 836 would give a positive G
- Messages
- 665
- Reaction score
- 13,607
- Points
- 503
wait there were 3 double bondscis trans was 3, actually there were 4 but two of the were same so total 3
so werent v supposed to have 8 isomers?
- Messages
- 24
- Reaction score
- 7
- Points
- 13
these are for benzene, for phenol just dilute HNO3 at rtp
i wrote dil. hno3 and h2so4 at room temp
is it wrong ?
- Messages
- 132
- Reaction score
- 74
- Points
- 38
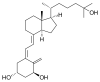
wait there were 3 double bonds
so werent v supposed to have 8 isomers?
Attachments
- Messages
- 132
- Reaction score
- 74
- Points
- 38
here is the compound guys the optical isomers are 6