- Messages
- 584
- Reaction score
- 555
- Points
- 103
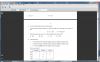
In options 1 and 2, the products are formed by electrophilic addition of DBr and alkene. Option 3 is not possible because when DBr reacts with alcohol in a nucleophilic substitution reaction the hydrogen in the OH is not replaced by D. So the answer is option 1 and 2, right? Correct me if I am wrong. Thanks for your help.refer to the organic reactions and see the HBr reactions, you'll find one that makes that benzene ring thing in option 1 and of course the halogenoalkane from option 2 .... option 3 however will not be selected because the H in the OH group is not replaced when a reaction happens, this whole group either goes or it doesn't change.... i hope i got it right.