- Messages
- 1,394
- Reaction score
- 1,377
- Points
- 173
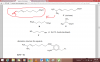
okaayy i got the one with H but not the a and b parts..well Na reacts with alcohol phenol and carboxylic acid right so given this fact there are 6 hydrogen available to be replaced with Na metal 6 hydrogen atoms. 2 atoms in one molecule so 3 moles .
for (a) alcohols dont react with NaOH so 3 will react
for (b) again 2 OH of phenols and 1 OH of carboxylic acid will react but this time there would also be base hydrolysis so total 4 moles of NaOh required
if alcohols dont react then with which parts of the compound will 3 NaOH react?
same with the b part....
ummm are u saying that the phenols and carboxylic acid will react in a and b parts but alcohols won't ?