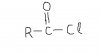- Messages
- 1,394
- Reaction score
- 1,377
- Points
- 173
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w12_qp_42.pdf
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w12_ms_42.pdf
In Q5, is it wrong to write just reduction instead of redox in the type of reaction G will hav with Na metal? Also how it is redox? the way i see it G is getting reduced and that is it.... ?? is it bcz it is losing H and also becoming negative (ox. state) that it is called redox?
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w12_ms_42.pdf
In Q5, is it wrong to write just reduction instead of redox in the type of reaction G will hav with Na metal? Also how it is redox? the way i see it G is getting reduced and that is it.... ?? is it bcz it is losing H and also becoming negative (ox. state) that it is called redox?