- Messages
- 318
- Reaction score
- 336
- Points
- 73
Please Zaq helpIt should be D. Why not D
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s09_qp_1.pdf
Q3 Why not not DDDDDD!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Please Zaq helpIt should be D. Why not D
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s09_qp_1.pdf
Q3 Why not not DDDDDD!
if you notice it is in the 6th group and 2nd periodIt should be D. Why not D
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s09_qp_1.pdf
Q3 Why not not DDDDDD!
for the first four IE the difference is roughly 2000It should be D. Why not D
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s09_qp_1.pdf
Q3 Why not not DDDDDD!
Last trend such a big difference?" 2p6 is stable,if you notice it is in the 6th group and 2nd period
ther is only 1 element that is O(8)
1s2 2s2 2p3
Can you tell me how you think it is D?
Thats gud
we
for the first four IE the difference is roughly 2000
between the 4th and 5th there is a difference of 3550.but the principal quantum number is still the same i.e the principal quantum no is 2.
the distance between 2p and 2s is small.while distance between 2s and 1s is large
What do you meanLast trend such a big difference?" 2p6 is stable,
the first four ionization energies are for 2p4 electronswoh what i do is look for a huge change in the ionisation energy like here it is from 13300 to 71000
so basically the outer shell has 6 electron here cause the when we remove the 7th one is is most probably closer to the nucleus
and from the periodic table this element is in the 6th group
and they have mentioned from Li to Ne so 2nd period
What do you mean? yes it but where will you get those 2 extra electrons from this is for Ne
Smart girl!we
for the first four IE the difference is roughly 2000
between the 4th and 5th there is a difference of 3550.but the principal quantum number is still the same i.e the principal quantum no is 2.
the distance between 2p and 2s is small.while distance between 2s and 1s is large
Q1Thats gud
Got some (actually 6) q from m/j/06 :/
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s06_qp_1.pdf
Q1-D Q2-B Q10-C Q27-B Q38-B
1) 1 mol of N2O4 = 2 mol of NaOHThats gud
Got some (actually 6) q from m/j/06 :/
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s06_qp_1.pdf
Q1-D Q2-B Q10-C Q27-B Q38-B
That what i was trying to saythe first four ionization energies are for 2p4 electrons
the fifth and 6th are for 2s2
the last one is for 1s2
hence such a BIG difference because it is closest to the nucleus
the closer electron to the nucleus the more the IE
Q10Thats gud
Got some (actually 6) q from m/j/06 :/
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s06_qp_1.pdf
Q1-D Q2-B Q10-C Q27-B Q38-B
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_s04_qp_1.pdf
Can anyone explain question number 13 ans B, 28 ans D and 35 ans B.
Questions are;
In 13 why can't A be correct.
In 28 why is D correct, I think D is correct as tertiary alcohols don't oxidise even by strong oxidising agents right?
In 35 I don't get a point.
And in 19 is NO a reducing agent or not?
Q13 i have problems with equations too >.< but here i wouldn't have gone for A
lets look at other reaction of NaOH something you might be familiar with
like this one NaOH + НСl = NaCl + Н2О
why not NaClOH2?
Sulphur dioxide is acidic its just like HCl, it is weaker though
now you are left with B C or D
when acid+ base water is release
so not D and C isn't balanced
but i guess we need to learn this kind of equations
here is a link http://www.allreactions.com/index.php/group-1a/natrium/sodium-hydroxide just have a quick look
Q28 yes you are right
Q35
Carbon monoxide is neutral, and does not react like the other two acidic gases.
We could also just use the data booklet to determine/verify the element.That what i was trying to say

yup but that would take a while?We could also just use the data booklet to determine/verify the element.
..It will be given to us, right?The Ionization Energies page, I mean.
I dont get it.
wont the equilibrium shift to the right hand side if ammonia i.e product is removed?

Q1. Find out the number of moles of O2, since 1 mole of gas occupies 24000 cm^3 at RTP.
moles of O2 = 500/24 000
molecules of O2 = moles x 6.02 x 10^23 = (500/24000) x 6.02 x 10^23 = 1.25 x 10^22
Q2. Comparing the original structure to the resulting structure, we see that 5 C=C double bonds "gone".
So 5 moles of H2 are added.
If unable to visualise the above explanation, the diagram can show how H could be added in these locations.
View attachment 44133
Q7 and Q10
View attachment 44134
Q21. Is the answer really D? I got B.
View attachment 44136
Q22. The C=C undergoes oxidative cleaving, and the ends are oxidised to COOH.
View attachment 44137
Q23.
If confident, this question can be approached in a mathematical way
CnH(2n+2) + (3n+1)/2 O2 --> n CO2 + (n+1)H2O
when n increases, moles of O2 increases linearly. So its a line with a positive gradient.
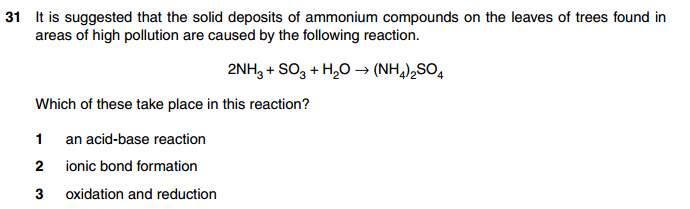
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now