- Messages
- 135
- Reaction score
- 85
- Points
- 38
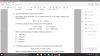
ThanksMoles of thiosulphate ions = 19.5 x 0.02 / 1000 = 0.00039mol
This is equal to moles of copper ions:
Moles of copper ions = 0.00039mol
Concentration of copper ions = 0.00039mol / 50 * 1000 = 0.0078mol/dm^3
This is concentration in moles per volume. Let's convert it to mass per volume:
0.0078mol/dm3 of copper ions = 0.0078*63.5 = 0.4953g/dm3
So there is 0.4953g of copper dissolved in every 1000cm3 of water.
We need to find percentage of copper by mass. Note that 1000cm3 of water has 1000g of mass.
Percentage by mass = 0.4953g / (1000 + 0.4953)g x 100% = 0.05%