- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
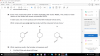
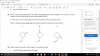
S2O7 + 2H2O ----> 2H2SO4 + (1/2)O2Use of the Data Booklet is relevant to this question.
The compound S2O7 is hydrolysed by water to produce sulfuric acid and oxygen only.
Which volume of oxygen, measured at room temperature and pressure, is evolved when 0.352 g
of S2O7 is hydrolysed?
A 12 cm3 B 24 cm3 C 48 cm3 D 96 cm3
answer is b how
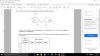
Moles of S2O7 = 0.352g / Mr = 0.001998mol
Moles of O2 evolved = 1/2 * Ans = 0.0009989mol
Volume = 24,000 * Ans = 24