- Messages
- 8
- Reaction score
- 2
- Points
- 3
ANY IDEA REGARDING THE PAPER TOMORROW? PLEASE PLEASE TELL.
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Not sure about the mean thing, but the rough titration is supposed to be above the titre values. So you might lose a mark there.can it be that the value of Rough titration to be 0.1 cm lower than the original titration . Annd will we lose mark of Not showing working while taking the mean of best titration. Anyone?
I heard organic is included, and the paper will be difficult than 33. Sorry.any guess for p34
Can someone help with this question ?
View attachment 63679
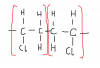
Easier way to do these kind of questions is to make a fully displayed formula of the structure which will help you to recognise the answer more easily
View attachment 63680
Thus, CH2=CHCl is the answer. option B
Can anyway explain this question or walk me through the answers ?
View attachment 63684
p.p1 {margin: 0.0px 0.0px 0.0px 0.0px; font: 11.0px Helvetica; -webkit-text-stroke: #000000} p.p2 {margin: 0.0px 0.0px 0.0px 0.0px; font: 11.0px Helvetica; -webkit-text-stroke: #000000; min-height: 13.0px} span.s1 {font-kerning: none}
A 0.005 mol sample of anhydrous calcium carbonate was completely thermally decomposed to give 100cm3 of gas measured at a certain temperature and pressure. In a separate experiment carried out at the same temperature and pressure, a 0.005mol sample of anhydrous calcium nitrate was completely thermally decomposed. The volume of gaseous products was measured. What total volume of gaseous products was produced from the calcium nitrate?
A 50cm3 B 100cm3 C 200cm3 D 250cm3
I had the same problem in this question when I solved this paper but now after repeated tries I was able to solve it correctly Alhamdulillah.
First find the value of the constant K as the angle of deflection is directly proportional to the charge mass ratio
PROTON:
Angle=+15 Charge= + mass= 1
15= k(+/1) thus K=+15
Option 1 :
Charge= +1-2= -1
mass= (atomic mass) 1+2=3
k=+15
Angle= (+15)*(-1/3)= -5..............option 1 correct
Option 2 :
Charge = +3-5 = -2
mass=3+3 =6
K=+15
Angle = (+15)*(-2/6) = -5..................option 2 is also correct
Option 3 :
Charge :4-1 = +3
mass=4+5 =9
K=+15
Angle = (+15)*(+3/9) = +5.......................Option 3 is incorrect.
Ans: B
The temperature is the same so it does not affectA large excess of marble chips was reacted with 25 cm3 of 1.0 mol dm–3 hydrochloric acid at 40 °C. How will the result be different when the reaction is repeated with 60 cm3 of 0.5 mol dm–3 hydrochloric acid at 40°C?
A The reaction is faster and less of the products are made.
B The reaction is faster and more of the products are made.
C The reaction is slower and less of the products are made.
D The reaction is slower and more of the products are made.
Answer is D plz help I dont understand the part more product
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now