-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
chemistry
- Thread starter Safia05
- Start date
- Messages
- 676
- Reaction score
- 111
- Points
- 53
That's the Biochemistry part of applications in Chemistry, Best is refer to the A level Bio text book, Mary Jones preferably.
- Messages
- 1,148
- Reaction score
- 168
- Points
- 73
Its in the data booklet:
http://xtremepapers.net/CIE/Internation ... let%29.pdf
http://xtremepapers.net/CIE/Internation ... let%29.pdf
- Messages
- 1,148
- Reaction score
- 168
- Points
- 73
See when the equation says iodine and tin metal then use the equation where Sn metal is present. If the question says Sn ion, use the equation where Sn ion is present.
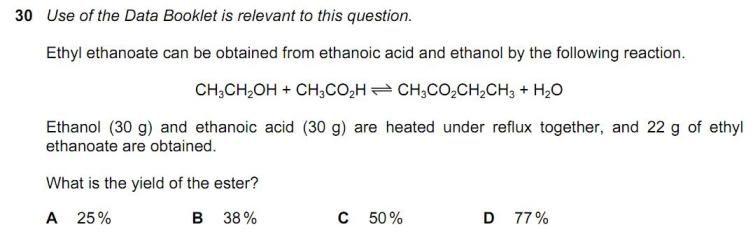
How to calculate [Pb 2+]? This is from nov 2011 P41. Thank you in advance!
destined007 said:See when the equation says iodine and tin metal then use the equation where Sn metal is present. If the question says Sn ion, use the equation where Sn ion is present.
There are cases where the keywords are not mention. What should I do then?
- Messages
- 1,148
- Reaction score
- 168
- Points
- 73
Ksp=[Pb+].([Cl-1])^2 can be seen from the equation
if x is the concentration of Pb, 2x will be the concentration of Cl. 1:2 ratio
2x10^-5= X . 4X^2
X=cube root((2x10^-5)/4)
if x is the concentration of Pb, 2x will be the concentration of Cl. 1:2 ratio
2x10^-5= X . 4X^2
X=cube root((2x10^-5)/4)
- Messages
- 1,148
- Reaction score
- 168
- Points
- 73
Tell me the question where you can't figure out which equation to use.