- Messages
- 772
- Reaction score
- 149
- Points
- 38
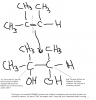
Hello. I'm a bit confused on one thing, that when we oxidize an alkene like propene we first get a secondary alcohol, and then on further heating we can get an aldehyde and then an acid. And for 2-methyl-prop-1-ene, firstly we get a tertiary alcohol, and then a ketone. Just wanted to ask that ain't primary alcohols oxidized to aldehydes, and secondary alcohols oxidised to ketones? Please explain it fully! =s