- Messages
- 68
- Reaction score
- 288
- Points
- 83
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
1c(ii)
hmm..srryBroken link.
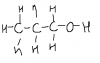
4 (a)(i)Refer 2 d periodic tbl 4 d valencies-- Sc3+, F-http://www.xtremepapers.com/papers/CIE/Cambridge IGCSE/Chemistry (0620)/0620_w11_qp_32.pdf
Also,
4a (i)
4b (i)
5c
5d
6c
7c
Please need help, I'm bad in chemistry, also still waiting for answer about my past doubt.
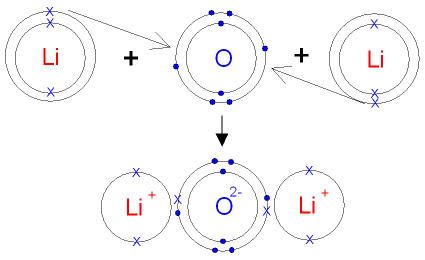
4 (a)(i)Refer 2 d periodic tbl 4 d valencies-- Sc3+, F-
so there will b 3 F to make d compound:: ScF3 Draw a circle of Sc3+, no e- around it. draw three F atoms, 7 o's and 1 x around each.
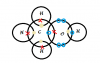
(b)(i) agan valency in PT... Si is 4, O is 2.. Cross them, Si2O4... thus, draw 4 O around evy Si n 2 Si around evy O
5(c) methanol, 1 C atom... CH3OH
C has 4 valency electrons, draw dat
around d C, 3 H n 1 O....
beside d O, one more H (4m OH) Refer 2 d file uploaded
(d) when propanol is oxidised, u get propanoic acid
C3H5COOH
evy C must have 4 bonds, O 2 bonds, H ONLY 1 bond
H H O
| | ||
H--C---C---C--O--H
| |
H H
(ii) u want it 2 b oxidised, leave it open in air (dat contains Oxygen!) n u want it by fermentation, so use microbes/bacteria, etc.
6(c) lo! behold! Stoichiometry....
moles of BaSO4: 1 mol has a mass of 233g, n in reality, 1.398 g r 4med.. so x will give u 1.398g. unitery method--233/1.398 = 0.006
moles fo MgSO4.xH2O: refer 2 d eqn....1 molecule gives 1 molecule, so 0.006 will (sensible, hmm..) give u 0.006, rite?
they've given u d mass of hydrated mg sulfate..1.476....so again 0.006 gives 1.476, so 1 will give u 1.476/0.006 = 246
the anhydrous thing is 120 g so 4m 246, how much is water? i think 126?
Moles = mass/Mr = 126/(16+2(1)) = 126/18 = 7
hp u got it....
7(c) 0.026 mols of Pb + 0.104 mols of C2H5 = ur prod
so 1 mol of Pb(refer 2 d qn, only 1 atom or whatever is there) will use 0.104/0.026 C2H5 = 4......thus n=4
hope u got it..sorry 4 being so bad at xplaining
all d best, n u'll do good, wid the Lord's Help, 4 its only He who CAN help.....
6c- the num of baso4 formed is 0.006 cuxhttp://www.xtremepapers.com/papers/CIE/Cambridge IGCSE/Chemistry (0620)/0620_w11_qp_32.pdf
Also,
4a (i)
4b (i)
5c
5d
6c
7c
Please need help, I'm bad in chemistry, also still waiting for answer about my past doubt.
Yes but depends on the one u use with uv light u get black spot n with resorcinol and ninhydrin colouredlocating agent in chromatography shows u d clr rite?
6c- the num of baso4 formed is 0.006 cux
Mass of barium sulfate formed = 1.398 g
SO n=m/mr so 1.398/233 = 0.006
The number of moles of MgSO4.xH2O = 0.006 because both are 1 ;/ i dunno how to explain :/
The mass of one mole of MgSO4.xH2O = as we know Mass of hydrated magnesium sulfate = 1.476 g
so, n= m/mr......0.006= 1.476/mr
so mr= 246g
The mass of xH2O in one mole of MgSO4.xH2O = as we know The mass of one mole of MgSO4 = 120g
so, 246-120= 126g
x= 7 because 126/18
and we got 18 by H*2+16
so n= m/mr
q7c u jus have to divide 0.104/0.026... i dunno y but i got the answer by checkin the valency from periodic tabel
the answer for others are attached except 5c i dunno tht one
EDIT- the third drawing is wrong it has to be alcohal IGNORE IT
lithium oxide has the electronic configuration of 2,1 so it has 1 electron in its outer shell that it can loose or give some other non metal such as oxygenCan you explain how you did this? I got some idea, but still need elaboration.
Thanks in advance.
Increase in temp causes equilibrium to shift to the endothermic sidehttp://www.xtremepapers.com/papers/CIE/Cambridge%20IGCSE/Chemistry%20(0620)/0620_w11_qp_33.pdf
6 (b)(i)
Plz xplain all d things dat affect equilibrium..
how do u noe dat its endo or exo? in d qn, its 200 degree C, so i said exo, n it was wrong..Increase in temp causes equilibrium to shift to the endothermic side
increase in pressure causes the equilibrium to shift to the sides with fewer moles
no dear nothing tricky jus look at the equation! like eg- 2CO2+C= 3CThank you, but in The number of moles of MgSO4.xH2O and mass of one mole of MgSO4.xH2O, is there something tricky? don't you need the mass of one mole to find the number? but the question asks to find the number of moles first and not the mass of one mole.
i think so. those dat can vary must b kept constantwhat is constant variables in paper 6 physics ? is it temperature of surrounding/thermometer/ and conc. of volume ?
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now