View attachment 41174
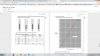
what would be the second intersecting line to plot on graph other than rise in temperature? weird question
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
View attachment 41174
what would be the second intersecting line to plot on graph other than rise in temperature? weird question

woah thanks
but where is the intersection point
the point where the graph bends.but where is the intersection point
e) if 25.9 --> 0.0025 molesView attachment 41167
View attachment 41169
View attachment 41170
can somebody plz eplain this question from part e onwards ...
Hosla.Hows your preparation going?
Yar aap jaise log kaise itney genius hote ho.
Na kabhi yeh experiment dekha hai na suna.View attachment 41172
FIRST PART OF EXPERIMENT
Blow the air in the test tube with the help of a blowing-pump. This is the air you inhale.
When the water will be displaced down and air will come in contact with potassium, it will burn using the OXYGEN gas present in air. The water level will rise.
Measure this rise in water level, 'A'
SECOND PART OF EXPERIMENT
(Set up the same apparatus and) Blow the air in the test tube with your mouth. This is your exhaled air.
Potassium will burn again when it comes in contact with air, using the OXYGEN, and the water level will rise.
Measure this rise in water level, 'B'.
We will observe that A > B because inhaling air contains more oxygen.
HEY! WAIT! ISNT IT THAT POTASSIUM BURNS IN WATER TOO? IF IT IS, THEN I GUESS WE SHOULD USE OIL INSTEAD OF WATER
Actually both lines need to be extended to make the point of intersection clear which is important.but where is the intersection point
See, if oxygen is absorbed from the air, the level of the air decreases to which the liquid responds but this is only possible if we have something to absorb CO2 otherwise the level would remain the same as the CO2 would compensate for the oxygen used up.So whats the right explanation
Night probably.This is the last day.Guys if you have any tips for BIO ATP please share
funky brat Awesome12 ***amd*** Dark Destination
robinhoodmustafa Can any of your A* holders and xpc users before this batch help?
which two lines? the horixontal n diagonal? how did you plotted the horizontal one?Actually both lines need to be extended to make the point of intersection clear which is important.
I did say to have a substance to absorb CO2.Read my answer again:See, if oxygen is absorbed from the air, the level of the air decreases to which the liquid responds but this is only possible if we have something to absorb CO2 otherwise the level would remain the same as the CO2 would compensate for the oxygen used up.
I've done this paper before. I haven't read the question but I remember when you'll plot the points you'll easily find these 2 lines from your plots then extend both in a direction that they meet each other.which two lines? the horixontal n diagonal? how did you plotted the horizontal one?
Hmm I missed it then, sorry.I did say to have a substance to absorb CO2.Read my answer again:
''So there might be some error in your question.Going by your idea,i say that place some germinating seeds in a tube over a guaze with soda lime beneath it to absorb co2.Attach a tube with a bubble as the miniscus.As the seeds respire they use up the o2 present in the tube and bubble moves to the right side.We can repeat this experiment with a substance to absorb O2 and show that no change in bubble level is seen.thus seeds use 02 to respire. ''
no, thats enough...thankse) if 25.9 --> 0.0025 moles
1000 (1dm^3)---> x
25.9x= 2.5
x= 0.0965 moles
Then the formula no. of moles= mass in g/mr
0.0965= 8.50/x
x=88 g
Now seee this CnH2n+1COOH
Total mass= 88g.
COOH= 45g
CnH2n+1= 88-45
= 43g
C3H2(3)+1= 43g.
Therefore the formula is= C3H7COOH.
Need help with the next part?
So it is right thenHmm I missed it then, sorry.
just clear which quantities you plotted? the rise in temp and...?I've done this paper before. I haven't read the question but I remember when you'll plot the points you'll easily find these 2 lines from your plots then extend both in a direction that they meet each other.
Yeah, you may clap for yourself.So it is right then
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now