- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Can anyone PLEASE explain these question!
Q5,7,17,18,24,25,30,33,34 from
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w05_qp_1.pdf
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w05_ms_1.pdf
I could answer 5,6,25,30,33 if u figure out the rest plz tell me how to solve them as well!
W05qp1
Q17. This question comes from the chapter of transition metals, I'm not sure its relevant to your syllabus now.
Q18. ammonium salt + base --> salt + water + NH3
ammonium sulfate + calcium hydroxide --> calcium sulfate + H2O + NH3
Nutrient of nitrogen in the fertilzer is lost as NH3 gas.
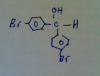
Q24. Three possible alkenes
cis -but-2-ene, trans-but-2-ene and but-1-ene