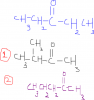- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
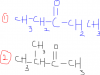
moreover, we just have to look if the total moles at one side are equal to moles on the other side for this method? We donot have to consider the individual moles of compounds right? And this methold is true for all the reactions or only reversible?