- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
yea right, but I'm talking about ENTHALPY CHANGE OF COMBUSTION of H2For enthalpy change of formation, the arrow must point from the individual constituents to the final product. If the arrow is in the opposite direction, the enthalpy change will have the same magnitude but opposite direction.
oh.. I get your point but why not B?
yea right, but I'm talking about ENTHALPY CHANGE OF COMBUSTION of H2
But don't all molecules have van der waals forces?Iodine: Covalent and van der waals forces
Silicon dioxide: Giant covalent
Sodium chloride: Ionic
Zinc: Metallic
Oh.. so it shud have been +2* enthalpy change of combustion? am I right?That's exactly the same. Recall the definition of combustion and you should realise that the arrow should be pointing toward the product of combustion.
Oh.. so it shud have been +2* enthalpy change of combustion? am I right?
Ratio is 3:2somebody solve me this pleasee
All MOLECULES. Silicon dioxide, sodium chloride, and zinc have a giant lattice structure. They are NOT molecules.But don't all molecules have van der waals forces?
Oh.. so it shud have been +2* enthalpy change of combustion? am I right?
Initial: b, 0, 0Help please??
View attachment 54318
I think it might be because if the pH is 14, there is a very high concentration of hydroxide ions. This would lead to OH- ions having high chances of collision with the aluminum and barium nitrate. It's just a guess I'm not sure thoughhttp://onlineexamhelp.com/wp-content/uploads/2014/08/9701_s14_qp_11.pdf
Guys , in Q8 - we are choosing ph 7 becaz the products are basic in nature ?
but this reaction isnt equilibrium related to do the shifting :/
Which molecules, each with a linear carbon chain, can have an optically active isomer?
I - C3H6BrI
II - C3H4BrI
III - C3H6I2
IV - C3H4Br2
A. I and II only
B. I, II and III only
C. II and III only
D. I, II and IV only
answer is B, explain?
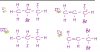
Carbon monoxide burns readily in oxygen to form carbon dioxide.
What can be deduced from this information?
1 The +4 oxidation state of carbon is more stable than the +2 state.
2 The standard enthalpy change of formation of carbon dioxide is more negative than that of
carbon monoxide.
3 The value of the equilibrium constant for the reaction, 2CO(g) + O2(g) 2CO2(g), is likely to
be high.
answer is A, i need to know why 3 is correct? explanation please!
What's the answer?Help please??
View attachment 54318
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now