- Messages
- 456
- Reaction score
- 280
- Points
- 73
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
In free radical substitution any C-H bond can undergo, homolytic fission.View attachment 59685
Ummm I didn't even know branched chains were possible in free radical substitution.Can someone explain how the branched chain will form and why the answer is B (1 and 2 only)
Thanks a lot !In free radical substitution any C-H bond can undergo, homolytic fission.
In 1, the C-H of Carbon 1 of one molecule has undergone this, and the Carbon 2 on the other molecule has undergone homolytic fission.
Similarly, in 2, both molecules' C-H of carbon 2 has undergone this.
But in 3, there are not two molecules of propane. But actually one molecule butane, and other of ethane. So this not correct as the question asks about the termination step in bromination of propane.
View attachment 59686
I have drawn boxes around the two free radicals which are involved in termination steps.
The bond dissocation energy is relevant to gasesous reactants.View attachment 59687
I think both A and D are possible answerstandards but it's D in the ms. :/
Oh didn't think of that! ThanksThe bond dissocation energy is relevant to gasesous reactants.
I was doubting it so I googled it. Must not take all the credit.Oh didn't think of that! Thanks
Haha I appreciate your honestyI was doubting it so I googled it. Must not take all the credit.
THANK YOU != (Mass no x Relative Abundance) / (Relative Abundance)
= {(10 * 1) + (11 * 4)} / 1 + 4
= 10.8
There is no peak for the mass no. 12, so we don't need to consider it. Note: Even if you consider it, it will be 12 * 0 = 0
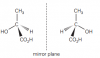
Maybe they have drawn two possiblities. If u see that other part is not optical isomer. So its two possiblities, either optical isomer of left or of rightView attachment 59711
in 2014 may/june paper 42 mark scheme the optical isomers are given like this.
View attachment 59713
In the syllabus, it's given that potical isomers should be drawn like this.
My question is in the mark scheme why is the OH as a dashed line and then in the second isomer as a wedge but in the syllabus it is not ?
Both are fine. Key thing is to make sure the diagrams show the two molecules are non-superimposable.View attachment 59711
in 2014 may/june paper 42 mark scheme the optical isomers are given like this.
View attachment 59713
In the syllabus, it's given that potical isomers should be drawn like this.
My question is in the mark scheme why is the OH as a dashed line and then in the second isomer as a wedge but in the syllabus it is not ?
[PCl4]+ will be a tetrahedral structure, like CH4 or NH4+. I think showing the dative bonds is not important, but you could show one of the Cl atoms receiving a pair of electrons from P. (Just like N donates a lone pair to H in NH4+)Are these complex ions ? and if so when we draw the structure how do we draw the dative bonding ?
View attachment 59722
How do we know if it is tetrahedral or square planar ?[PCl4]+ will be a tetrahedral structure, like CH4 or NH4+. I think showing the dative bonds is not important, but you could show one of the Cl atoms receiving a pair of electrons from P. (Just like N donates a lone pair to H in NH4+)
[PCl6]- will be an octahedral structure, like SF6. Again the coordination of the bonds shouldn't be important but you could show one Cl atom donating a pair of electrons to P.
I'm guessing your trouble is with the right column.Anyone? The ans is A.View attachment 59700
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now