- Messages
- 438
- Reaction score
- 106
- Points
- 53
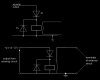
you are absolutely right that it provides evidence for particulate nature of light.
but are you sure it doesnt show the particulate nature of electrons too?
For example if there is a question that asks to design an experiment to prove particulate nature of electrons, What experiment do we draw?
Wikipedia :- "The photoelectric effect requires photons with energies from a few electronvolts to over 1 MeV in high atomic number elements. Study of the photoelectric effect led to important steps in understanding the quantum nature of light and electrons and influenced the formation of the concept of wave–particle duality"
Are you sure electrons are not proven as particles from the photoelectric effect?
okay first of all.. we study wave-particle duality in our syllabus which is about light. That's it. Just how light acts as a wave and a particle.
but.. Experiments with a Crookes tube first demonstrated the particle nature of electrons. And the wave nature of electrons comes from the De Broglie hypothesis.