- Messages
- 971
- Reaction score
- 532
- Points
- 103
Haha, yes.
http://www.scribd.com/doc/37574186/9701-w01-qp-2
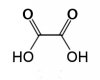
Nov 01, Q4 (c) (i) & (ii) and part (d)
What are the answers?
http://www.scribd.com/doc/37574186/9701-w01-qp-2
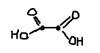
Nov 01, Q4 (c) (i) & (ii) and part (d)
What are the answers?