- Messages
- 49
- Reaction score
- 13
- Points
- 8
i was wondering about that in may/jun 2007 Q40( physics). we need to divide proton no. by nucleon no., n i don't get it why...Cn sm1 explain me the charge to mass ratio of a particle (e/m)
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
i was wondering about that in may/jun 2007 Q40( physics). we need to divide proton no. by nucleon no., n i don't get it why...Cn sm1 explain me the charge to mass ratio of a particle (e/m)
for Q24 its A since incomplete combustion of a hydrocarbon gives CO, while complete combustion gives CO2 and H2OSomeone help me please for these 3 questions from june 02
ThanksView attachment 15254
For question 23, i would have gone for C because the conversion of 1st compound to the second compound occurs as the C--------------Br and C-OH. you will need to add a further carbon atom, which is achieved by the CN. and finally convert to carbocylic acid by hydrolysis.Someone help me please for these 3 questions from june 02
ThanksView attachment 15254
For question 23, i would have gone for C because the conversion of 1st compound to the second compound occurs as the C--------------Br and C-OH. you will need to add a further carbon atom, which is achieved by the CN. and finally convert to carbocylic acid by hydrolysis.
For question 24 i would have gone for A because the complete combustion of C is CO2 not CO and NO is not a result for incomplete combustion of Hydrocarbon.
For the last question i would have gone for A as the central carbon would bear 4 different constituents upon reaction with CN-
Hope my replies helped.
Its okay mateThank you very much Amy Bloom ! That's sooo humble from you
I'm not so sure maybe smzimran can confirm this:Plz xplain me charge to mass ratio
Can anybody help me with this. i need an answer by tomorrow.
Ameen. Yeah thanks mate, at least for trying. the parts for which i was stuck you solved it. thanks loads.PART A: NaOH and HCl are in 1:1 ration hence you calculate moles for HCl and use it to find the conc of NaOH
so for HCl, n = (0.1 x 25.45)/1000 = 2.55 x 10 ^-3
so for NaOH, 2.55 x 10 ^-3 = ( c x 25)/1000 = 0.102 moldm-3
PART B: H+ and OH- in 1:1 ratio
so for HCl, n = (0.1 x 17.3)/1000 = 1.73 x 10 ^-3
so for OH-, 1.73 x 10^-3 = ( c x 25)/1000 = 0.0692 = 6.92 x 10 ^-2 moldm-3
PART C:
PART D: (total OH- conc calcualted in B) - (conc of OH- from NaOH calculated in C)-----> (6.92 x 10 ^-2) - (0.051)
= 0.0182 moldm-3
PART E:
PART F: Ksp = [Ca2+] [OH-]^2
= (9.1 x 10 ^-3) (6.92 x 10 ^-2)^2
= 4.36 x 10 ^-5 mol3 dm-9
sorry couldn't do c and ebut i have used their answers for the next qns.
Hope it helped inshaAllah
you are welcome anytimeAmeen. Yeah thanks mate, at least for trying. the parts for which i was stuck you solved it. thanks loads.
part a.) sign is negative coz bond formation is exothermic and releases energy, hence the negative signhey so i have a question here of november 2007 question 4 part b
http://www.xtremepapers.com/papers/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w07_qp_4.pdf
can anyone explain me why in part(a) the sign is negative
and in part(b) why we are not taking the value of cl2
thankyou
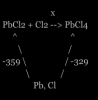
part a.) sign is negative coz bond formation is exothermic and releases energy, hence the negative sign
part b.) we use hess's law so basically we have the 2 enthalpy of formation for the PbCl2 and PbCl4 so we make and equation
so we get a triangle kind of thing,
View attachment 15488
soooo, you follow the directions of the arrows, arrows in one direction, you add 'em, those in diff directions, you put 'em on the other side of the equal sign
-359 + x = -329
hence, x = 30
Hope that helped

alhamdulillah...thanx
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now