- Messages
- 869
- Reaction score
- 374
- Points
- 73
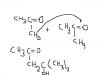
question 4 a) last box u have CH2(OH)CH(OH)CO2H u have a hydroxyl group attached to a carbon which is attached to one neighboring carbon and a hydroxyl group attached to a carbon which is attached to 2 neighboring carbons so a primary and a secondary alcohol present so when oxidation takes place the primary side will be a carboxylic acid which is this one CH2(OH)-R will be CO2H-R and this one CH(OH)CO2H is a secondary alcohol so will oxidise to a ketone and will be R-COCO2H add them together and u will get the organic compound HO2COCO2Hhttp://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s09_qp_2.pdf
Variant 1, Q.4 a the last box when you add k2Cr2O7/H+. Also b)ii)
also Q.5. a)
5a u have 2 similar ketones CH3-C=O(CH3) that will be added to each other, one ketone will donate its H from a methyl group to the C=O of the other ketone and attach to each other..its really difficult to explain this one here but if u dont get the idea tell me....