- Messages
- 1,476
- Reaction score
- 1,893
- Points
- 173
AoA,
Q9:
pressure is directly proportional to number of moles of solute, as from the equation moles of solute are increasing pressure increases
, and it is obvious that glucose and fructose are not optical isomers so they are structural isomers
Therefore, the answer is D
Q10:
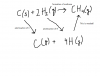
Thus, A is the answer!
Q35:
When firework is lit, heat is given to barium nitrate and it decomposes due to heat
Ba(NO3)2 --> BaO + 2NO2 + 0.5O2
BaO can be reduced to barium by Mg, and in doing so MgO will form
However, since the nitrate is not stable to heat, magnesium nitrate cannot form!
That means options 1 and 2 are correct and 3 is wrong
That is why B is the answer!
Q39:
You should know from the knowledge of reactions why options 2 and 3 are correct!
1 is incorrect because by boiling CH3CH2Cl under reflux with dilute alkali will produce CH3CH2OH
but adding mineral acid will not turn it into ethanoic acid, that can only be done by oxidation under reflux!