- Messages
- 7
- Reaction score
- 13
- Points
- 13
Q9. I usually try to create the eqm table
Ag+ .........+ ......... Fe2+.........--> .........Ag.........+ Fe3+
1...............................1...............................-..............0.... initial conc
-0.56........................-0.56................. -..........+0.56.... change conc
0.44...........................0.44...................- .............0.56.... final conc
Common student mistake: It is important to realise that Ag is a solid, so we do not fill in any info for it.
Refer to the steps below if you are not sure how the info in the above table is filled up.
1st step : Fill in info for the initial concentration
2nd step Fill in info that Ag+ is 0.44 moles for final concentration
3rd step: fill in the info in green
4th step: fill in the info in yellow
5th step: fill in the row for final concentration
Kc = [Fe3+]/[Ag+][Fe2+]
= (0.56)/(0.44)(0.44)
= 2.89
Q18. The reaction is Ca(OH)2 + SO2 --> CaSO3 + H2O
Q36.
X is N2 (element)
Y is NO (N2 + 0.5 O2 --> NO2)
Z is NO2
It might be tempting to think of X as C, but C does not really exist in the form of carbon element in the engine, but rather as part of a hydrocarbon fuel.
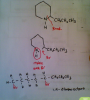
I'll try to explain this more with diagram when I figure out what is the best way to attach my sketches to a post.
For now, understand that using the original dibromine to react with NH3
NH2 will replace one of the Br
then the NH2 will join to the carbon that contains the other Br. The Br is removed and a ring is formed.
So, if we work backwards from the structure of coniine
1) cut the single bond between N and C, thus opening up the ring
2) C is now missing a bond, add a Br to it.
3) Replace the N-H group with a Br
4) That is the original structure of X
Alright thank you soo much!
For no. 9, I used to correct method, just that i calculated the mol for Ag (solid) as well, so i got the incorrect answer.